Menu
In today’s intricate healthcare landscape, the evaluation of patient data is often compartmentalized, restricting analysis to one modality at a time. This segmented approach presents a substantial challenge in identifying cohorts of metastatic non-small cell lung cancer (NSCLC) patients. DEEP-Lung-IV represents a global effort to tackle this challenge. It is a multicentric and multimodal observational study focused on unraveling intricate signals linked to treatment responses and prognosis in stage IV NSCLC. By integrating and analyzing clinical, biological, genomics, and radiomics biomarkers, we aim to unlock predictive signatures and precise and invaluable insights from longitudinal study subject data.
Study type: Observational
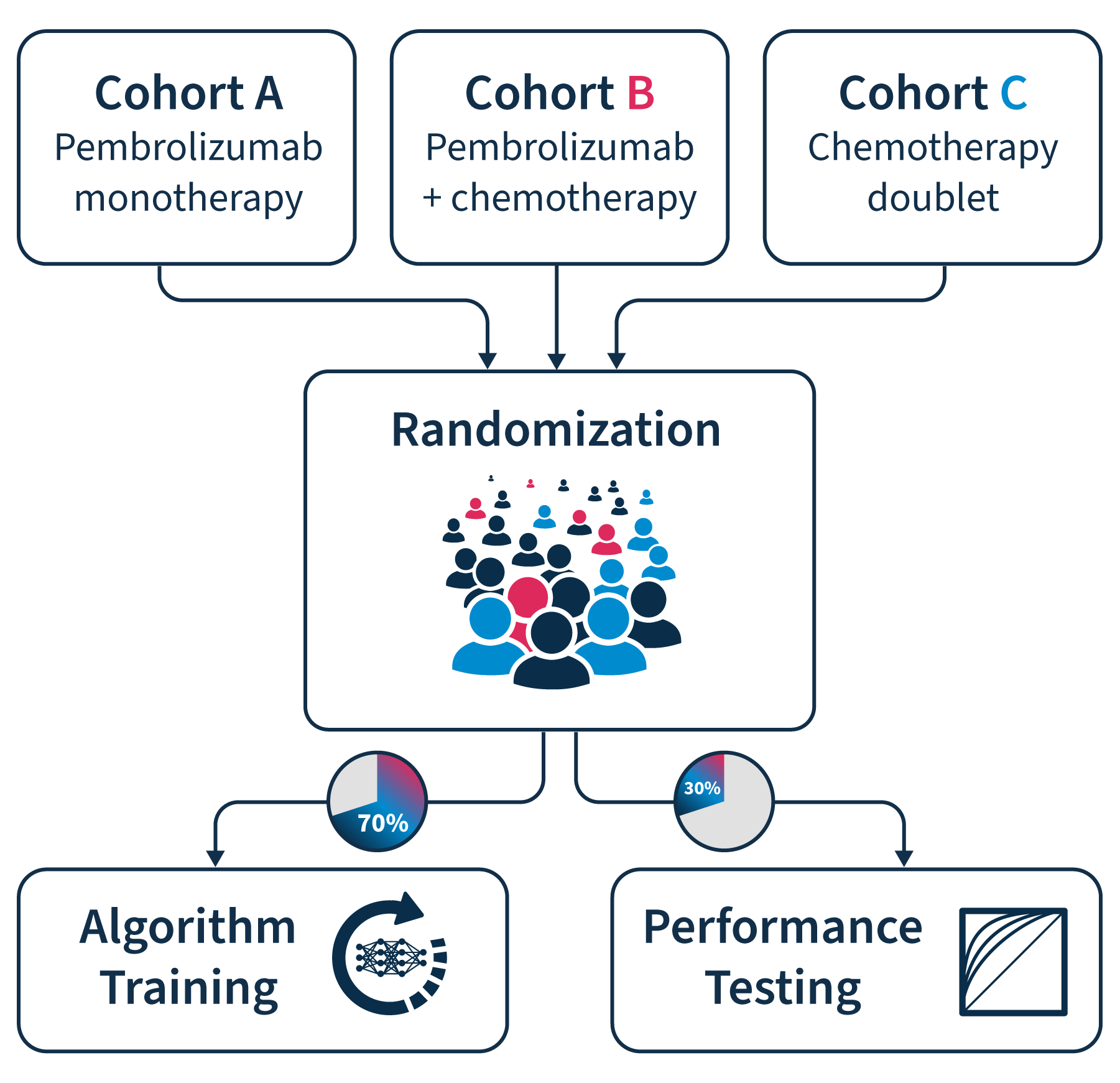
Cohorts:
• Pembrolizumab monotherapy
• Chemotherapy
• Combination therapy
Clinical Trials Identifier: NCT04994795
Start date: July 16, 2021
Study Sponsor: SOPHiA GENETICS
International study coordinator:
Jacques Cadranel


∼2,000
30
8





SOPHiA GENETICS products are for Research Use Only and not for use in diagnostic procedures unless specified otherwise.
SOPHiA DDM™ Dx Hereditary Cancer Solution, SOPHiA DDM™ Dx RNAtarget Oncology Solution and SOPHiA DDM™ Dx Homologous Recombination Deficiency Solution are available as CE-IVD products for In Vitro Diagnostic Use in the European Economic Area (EEA), the United Kingdom and Switzerland. SOPHiA DDM™ Dx Myeloid Solution and SOPHiA DDM™ Dx Solid Tumor Solution are available as CE-IVD products for In Vitro Diagnostic Use in the EEA, the United Kingdom, Switzerland, and Israel. Information about products that may or may not be available in different countries and if applicable, may or may not have received approval or market clearance by a governmental regulatory body for different indications for use. Please contact us at [email protected] to obtain the appropriate product information for your country of residence.
All third-party trademarks listed by SOPHiA GENETICS remain the property of their respective owners. Unless specifically identified as such, SOPHiA GENETICS’ use of third-party trademarks does not indicate any relationship, sponsorship, or endorsement between SOPHiA GENETICS and the owners of these trademarks. Any references by SOPHiA GENETICS to third-party trademarks is to identify the corresponding third-party goods and/or services and shall be considered nominative fair use under the trademark law.