Menu



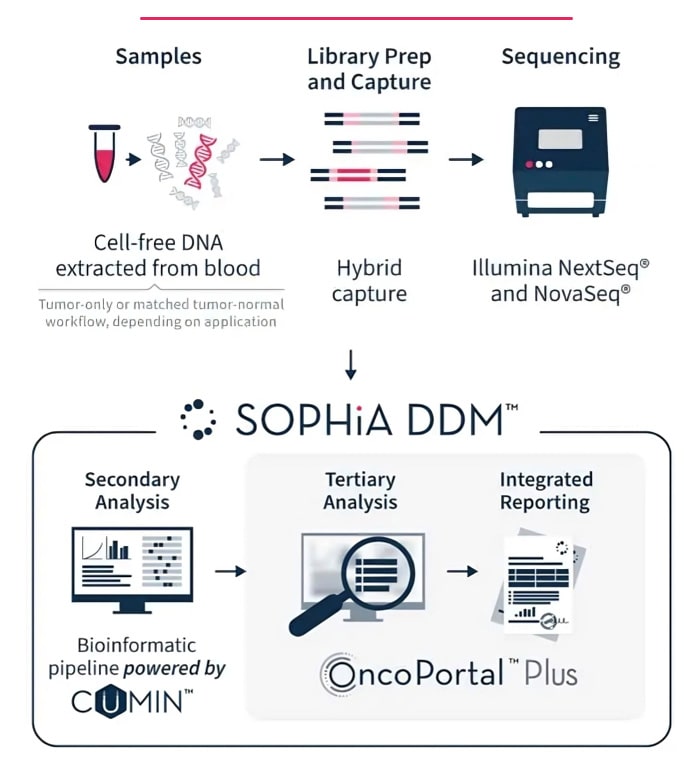
Use one universal, automatable protocol for robust sequencing across applications.
Precisely detect challenging variants with our proprietary algorithms.
Match cancer molecular profiles to current clinical data with OncoPortal™ Knowledge Base.
Quickly prepare comprehensive reports tailored to your needs.
Sensitive SNV and Indel detection
Accurate CNV calling
Partner-agnostic fusion calling
Universal genomic integrity assessment
Pan-cancer MSI detection
Precise molecular barcoding
Robust variant annotation






SOPHiA DDM™ enables sensitive detection of relevant variants at low allele frequencies (down to 5% VAF for FFPE analysis). By incorporating our proprietary CUMIN™ molecular barcoding technology into sample preparation and leveraging our bioinformatic expertise, errors introduced during library preparation, target enrichment, or sequencing can be filtered out and true variant alleles identified.
Technology Principles
Our robust annotation algorithm retrieves information from curated databases and uses de novo analytics to provide insights on the likely effects and pathogenicity of genomic variants. Coupled with SOPHiA DDM™ filtering capabilities, our accurate variant annotation facilitates the identification of relevant variants.
Technology Principles

| MSK-ACCESS® powered with SOPHiA DDM™ | Adapt existing SOPHiA GENETICS solid tumor applications to liquid biopsy | Custom liquid biopsy applications tailored to your needs | |
|---|---|---|---|
| Lorem ipsum | Lorem ipsum dolor sit amet, consectetur | Lorem ipsum dolor sit amet, consecdolor sit amet, consect | Proin dictum aliquam hendrerit. Quisque tortor nulla |
| Pellentesque nec | Lorem ipsum dolor sit amet, consectetur | Lorem ipsum dolor sit amet, consecdolor sit amet, consect | Proin dictum aliquam hendrerit. Quisque tortor nulla |
| Aenean eros orci | Lorem ipsum dolor sit amet, consectetur | Lorem ipsum dolor sit amet, consecdolor sit amet, consect | Proin dictum aliquam hendrerit. Quisque tortor nulla |
| Proin non mi diam | Lorem ipsum dolor sit amet, consectetur | Lorem ipsum dolor sit amet, consecdolor sit amet, consect | Proin dictum aliquam hendrerit. Quisque tortor nulla |
“Through this collaboration, we aim to enable the widespread application of precision medicine in oncology across Africa, and thus contributing to the improvement of patient outcomes across the African continent.
We believe our scientific expertise, combined with AI-enabled technologies and data-driven solutions enabled by SOPHiA GENETICS, presents a unique opportunity to fundamentally transform the journey of cancer patients through non-invasive cancer analysis, predictive genomic testing, and effective precision medicine”.

SOPHiA GENETICS products are for Research Use Only and not for use in diagnostic procedures unless specified otherwise.
SOPHiA DDM™ Dx Hereditary Cancer Solution, SOPHiA DDM™ Dx RNAtarget Oncology Solution and SOPHiA DDM™ Dx Homologous Recombination Deficiency Solution are available as CE-IVD products for In Vitro Diagnostic Use in the European Economic Area (EEA), the United Kingdom and Switzerland. SOPHiA DDM™ Dx Myeloid Solution and SOPHiA DDM™ Dx Solid Tumor Solution are available as CE-IVD products for In Vitro Diagnostic Use in the EEA, the United Kingdom, Switzerland, and Israel. Information about products that may or may not be available in different countries and if applicable, may or may not have received approval or market clearance by a governmental regulatory body for different indications for use. Please contact us at [email protected] to obtain the appropriate product information for your country of residence.
All third-party trademarks listed by SOPHiA GENETICS remain the property of their respective owners. Unless specifically identified as such, SOPHiA GENETICS’ use of third-party trademarks does not indicate any relationship, sponsorship, or endorsement between SOPHiA GENETICS and the owners of these trademarks. Any references by SOPHiA GENETICS to third-party trademarks is to identify the corresponding third-party goods and/or services and shall be considered nominative fair use under the trademark law.