Menu
Menu


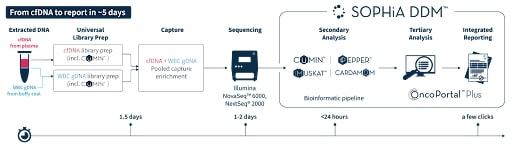
Have the option to sequence white blood cell DNA with cfDNA to filter CHIP variants.
Use one universal, automated protocol for robust sequencing across applications.
Precisely detect challenging genomic variants using our proprietary algorithms.
Match tumor genomic profiles to the most up-to date information with OncoPortal™ Knowledge Base.
Precise molecular barcoding
Sensitive SNV and Indel detection
Accurate CNV detection
Partner-agnostic fusion calling
Robust variant annotation




Our fusion calling technology accurately calls novel (partner-agnostic) fusions from DNA and leverages a probabilistic graphical model to reduce false positives. SOPHiA DDM™ can confidently identify very challenging mutations, such as KIF5B-RET fusion.

| MSK-ACCESS® powered with SOPHiA DDM™ - RUO | Community Liquid Biopsy Solution 21 genes – RUO | Adapt any existing SOPHiA GENETICS solid tumor applications to liquid biopsy |
|
|---|---|---|---|
| No. of genes | 147 genes curated by MSK experts |
21 genes curated from ESMO guidelines and expert insights |
LBx STS (42 genes), LBx HRS (16 genes), LBx ExtHRS (28 genes), and more |
| Cancer type | Across cancer types (NSCLC, prostate, pancreatic, biliary, bladder, breast & other cancers) |
Across cancer types (NSCLC, prostate, pancreatic, biliary, bladder, breast & other cancers) |
Solution-dependent |
| CHIP filtering | YES | OPTIONAL | OPTIONAL |
| Sequencer | Illumina NovaSeq™ X, NovaSeq™ 6000, NextSeq® 1000/2000 | Illumina NovaSeq™ X, NovaSeq™ 6000, NextSeq® 550/1000/2000 | Illumina NextSeq® 550/1000/2000 |
| Alterations | SNV/Indels, CNVs, gene fusions, TERT promoter, MET exon 14 skipping MSI (investigational) | SNVs/Indels, CNVs, fusions, MSI (investigational) | SNV/Indels (Depending on solution, CNVs, gene fusions, TERT promoter, and MSI may be available) |
SOPHiA GENETICS products are for Research Use Only and not for use in diagnostic procedures unless specified otherwise.
SOPHiA DDM™ Dx Hereditary Cancer Solution, SOPHiA DDM™ Dx RNAtarget Oncology Solution and SOPHiA DDM™ Dx Homologous Recombination Deficiency Solution are available as CE-IVD products for In Vitro Diagnostic Use in the European Economic Area (EEA), the United Kingdom and Switzerland. SOPHiA DDM™ Dx Myeloid Solution and SOPHiA DDM™ Dx Solid Tumor Solution are available as CE-IVD products for In Vitro Diagnostic Use in the EEA, the United Kingdom, Switzerland, and Israel. Information about products that may or may not be available in different countries and if applicable, may or may not have received approval or market clearance by a governmental regulatory body for different indications for use. Please contact us to obtain the appropriate product information for your country of residence.
All third-party trademarks listed by SOPHiA GENETICS remain the property of their respective owners. Unless specifically identified as such, SOPHiA GENETICS’ use of third-party trademarks does not indicate any relationship, sponsorship, or endorsement between SOPHiA GENETICS and the owners of these trademarks. Any references by SOPHiA GENETICS to third-party trademarks is to identify the corresponding third-party goods and/or services and shall be considered nominative fair use under the trademark law.