Menu
More than 50% of Acute Myeloid Leukemia (AML) patients relapse within three years after achieving complete remission.1 The SOPHiA DDM™ Residual Acute Myeloid (RAM) Solution utilizes targeted NGS analysis to accurately detect variants down to 0.01% variant allele frequency (VAF) for key genes linked to AML.


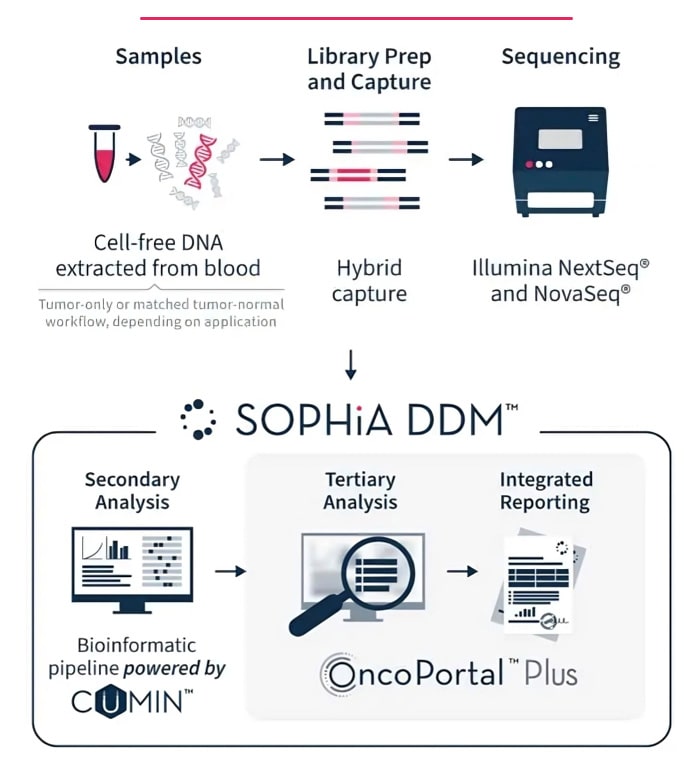
Use one universal, automatable protocol for sequencing on NextSeq® and NovaSeq™ systems.
Rely on multiple MRD markers to increase the gap between the signal and the noise.
Leverage a user-friendly interface that simplifies tracking of AML dynamics over time.
Quickly assess variants with enhanced tertiary analysis and community-driven insights.
Precise molecular barcoding
Sensitive SNV and Indel detection
Reliable MRD signal measurement
Analytics feature 4



“Through this collaboration, we aim to enable the widespread application of precision medicine in oncology across Africa, and thus contributing to the improvement of patient outcomes across the African continent.
We believe our scientific expertise, combined with AI-enabled technologies and data-driven solutions enabled by SOPHiA GENETICS, presents a unique opportunity to fundamentally transform the journey of cancer patients through non-invasive cancer analysis, predictive genomic testing, and effective precision medicine”.

SOPHiA GENETICS products are for Research Use Only and not for use in diagnostic procedures unless specified otherwise.
SOPHiA DDM™ Dx Hereditary Cancer Solution, SOPHiA DDM™ Dx RNAtarget Oncology Solution and SOPHiA DDM™ Dx Homologous Recombination Deficiency Solution are available as CE-IVD products for In Vitro Diagnostic Use in the European Economic Area (EEA), the United Kingdom and Switzerland. SOPHiA DDM™ Dx Myeloid Solution and SOPHiA DDM™ Dx Solid Tumor Solution are available as CE-IVD products for In Vitro Diagnostic Use in the EEA, the United Kingdom, Switzerland, and Israel. Information about products that may or may not be available in different countries and if applicable, may or may not have received approval or market clearance by a governmental regulatory body for different indications for use. Please contact us at [email protected] to obtain the appropriate product information for your country of residence.
All third-party trademarks listed by SOPHiA GENETICS remain the property of their respective owners. Unless specifically identified as such, SOPHiA GENETICS’ use of third-party trademarks does not indicate any relationship, sponsorship, or endorsement between SOPHiA GENETICS and the owners of these trademarks. Any references by SOPHiA GENETICS to third-party trademarks is to identify the corresponding third-party goods and/or services and shall be considered nominative fair use under the trademark law.