Menu
SOPHiA DDM™ is a leading IVDR-certified* genomics platform that leverages AI algorithms to accurately pinpoint signals within noisy, complex next-generation-sequencing (NGS) datasets. It seamlessly integrates into any genomics workflow to identify causative variants.
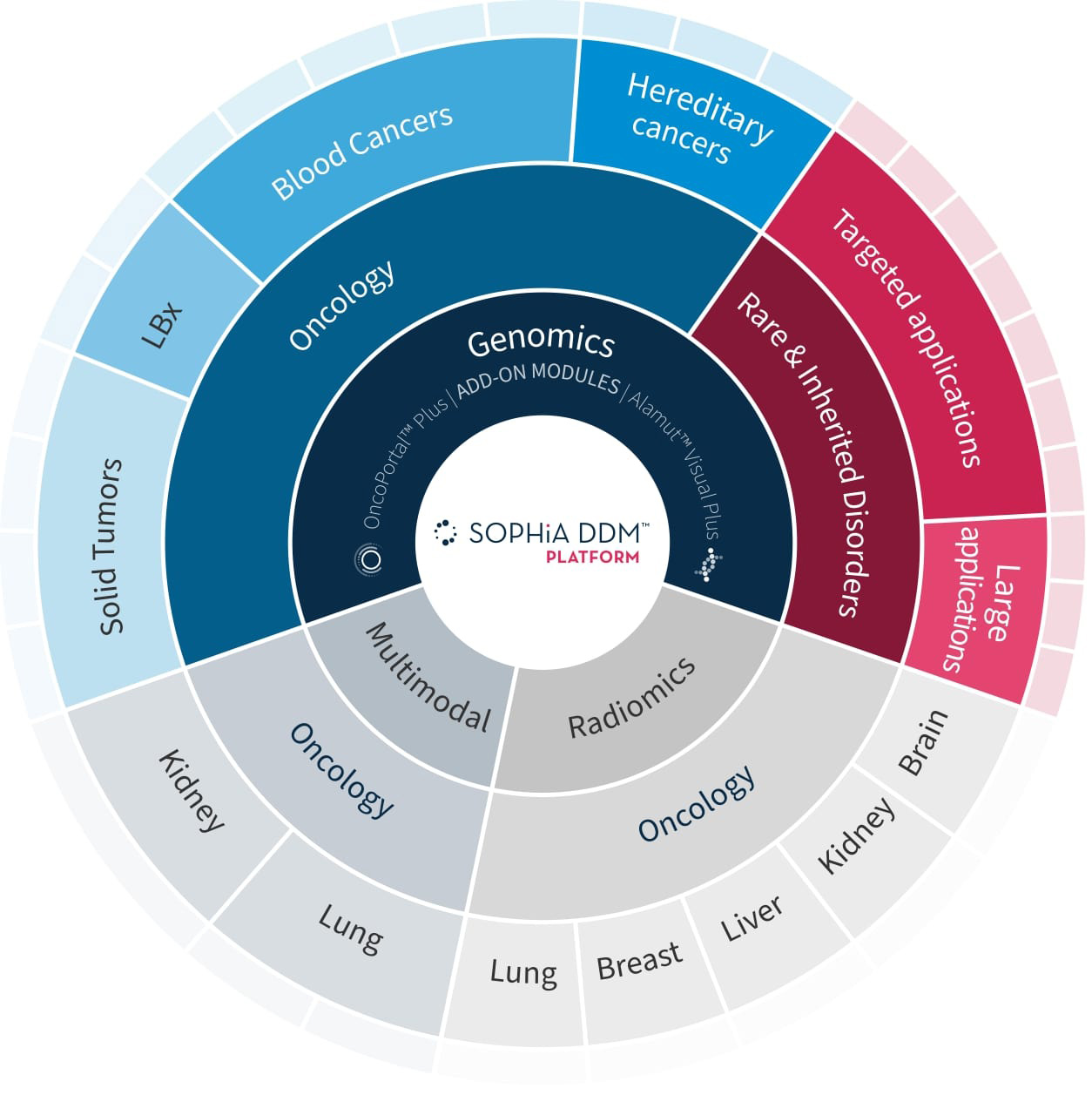
Dramatically expedite your interpretation by removing genomic variant analysis barriers with the SOPHiA DDM™’s annotation and pre-classification capabilities.
Add context to your variant interpretation. Flag variants and share insights with your disease-specific peer network or the entire SOPHiA GENETICS Community. Enhance your interpretation with direct access to the add-on modules OncoPortal™ and Alamut™ Visual Plus, that provide deeper, more comprehensive insights into your findings.
Watch how you can streamline your genomics workflow during this 4-minute tutorial video that walks through the key user-friendly features of SOPHiA DDM™ for variant analysis and interpretation.
IVDR certification validates the powerful analytical capabilities of the
SOPHiA DDM™ Platform for supporting patient diagnostics in the EU and beyond*. SOPHiA DDM™ is a single platform supporting IVD and RUO applications. Certification provides proof of compliance with regulatory requirements, reducing the burden for healthcare institutions when implementing in vitro diagnostic workflows.
*When used in Dx mode.
SOPHiA GENETICS products are for Research Use Only and not for use in diagnostic procedures unless specified otherwise.
SOPHiA DDM™ Dx Hereditary Cancer Solution, SOPHiA DDM™ Dx RNAtarget Oncology Solution and SOPHiA DDM™ Dx Homologous Recombination Deficiency Solution are available as CE-IVD products for In Vitro Diagnostic Use in the European Economic Area (EEA), the United Kingdom and Switzerland. SOPHiA DDM™ Dx Myeloid Solution and SOPHiA DDM™ Dx Solid Tumor Solution are available as CE-IVD products for In Vitro Diagnostic Use in the EEA, the United Kingdom, Switzerland, and Israel. Information about products that may or may not be available in different countries and if applicable, may or may not have received approval or market clearance by a governmental regulatory body for different indications for use. Please contact us at [email protected] to obtain the appropriate product information for your country of residence.
All third-party trademarks listed by SOPHiA GENETICS remain the property of their respective owners. Unless specifically identified as such, SOPHiA GENETICS’ use of third-party trademarks does not indicate any relationship, sponsorship, or endorsement between SOPHiA GENETICS and the owners of these trademarks. Any references by SOPHiA GENETICS to third-party trademarks is to identify the corresponding third-party goods and/or services and shall be considered nominative fair use under the trademark law.