The Trillium Health Partners Credit Valley Hospital Site in Mississauga and North York General Hospital in Toronto are part of the Ontario group of hospitals who developed a hereditary cancer NGS-based application in collaboration with SOPHiA GENETICS. Almost 2000 hereditary cancer samples have been analyzed using this fully integrated SOPHiA GENETICS workflow at the time of writing. Here, we highlight three complex variant cases kindly shared by Dr.’s Wang and Vaags, that were detected with the Ontario Hospitals’ SOPHiA DDM™️ Community Comprehensive Hereditary Cancer Solution (CHCS) powered by the SOPHiA DDM™ Platform.
Complex variant 1 – CNV in PMS2/PMS2CL pseudogene
Detection of variants in PMS2, one of the four mismatch repair genes associated with Lynch syndrome, is challenging due to the presence of pseudogenes.1 The SOPHiA DDM™️ PMS2/PMS2CL gene conversion module analyzes coverage in exons 11-15 of PMS2 and the corresponding exons of its pseudogene PMS2CL. In addition to viewing copy number variations (CNVs) within SOPHiA DDM™, the platform also provides a full CNV PDF report, which is particularly helpful for PMS2/PMS2CL pseudogene analysis. For each sample, PMS2 gene-like variants are visualized alongside the corresponding PMS2CL pseudogene-like variants. In this case, the Ontario group used the CNV report, which clearly suggested that there was a deletion in the real PMS2 gene as opposed to in the PMS2CL pseudogene.
The pseudogene pipeline really minimized reflex testing with long-range PCR.
Hong Wang, North York General Hospital, Toronto, Ontario, Canada
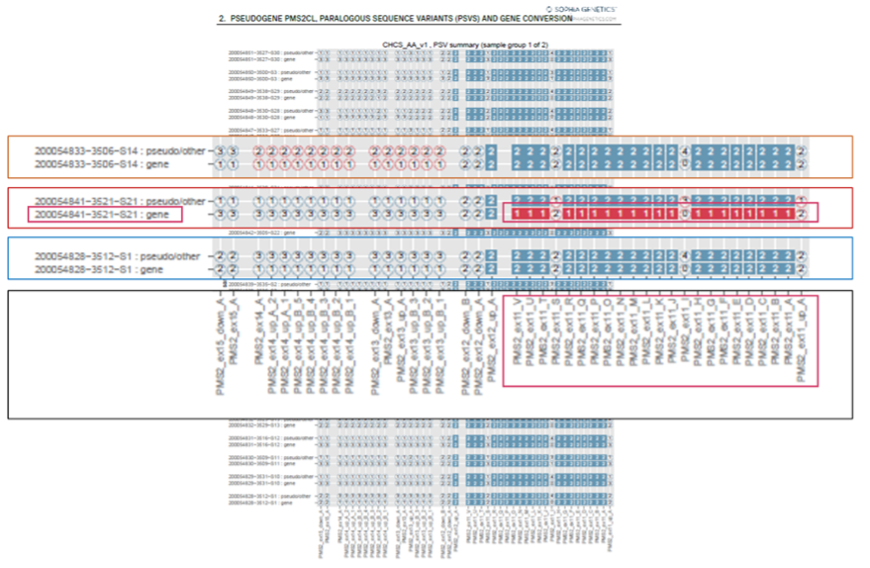
Figure 1. SOPHiA DDM™ pdf CNV analysis report showing a deletion in exon 11 of PMS2.
Complex variant 2 – CHEK2 low-level mosaic deletion
In the CNVs tab of the SOPHiA DDM™️ Platform, there is a visual report of the number of amplicons and their copy number for each gene. In this example from the Ontario group, although a deletion wasn’t called by SOPHiA DDM™ (which would be indicated by a red box containing a 1), the platform showed a medium confidence call for two exons in CHEK2 (Fig. 2A). The medium (versus high) confidence call was signified by two squares having a white instead of blue fill, representing an ambiguous copy number. When further investigating this finding using the CNV trace in SOPHiA DDM™, a shift from the normal copy number was apparent (Fig. 2B). SNP array indeed confirmed a deletion in exons 12 and 14 of CHEK2 in 30% of cells, representing low-level mosaicism.

Figure 2. Low-level mosaicism in exons 12 and 14 of CHEK2 shown in SOPHiA DDM™’s A. CNV tab, and B. CNV trace.
Complex variant 3 – SMAD4 processed pseudogene
In this final example, the Ontario group discovered multiple intronic deletions (introns 2, 9, 11, 5, 10, and 6) in SMAD4 via the SNVs/INDELs tab in SOPHiA DDM™ (Fig. 3A). In addition, the group reviewed the CNV trace to discover entire exon duplications across SMAD4 (except for exon 4; Fig. 3B). In combination, these findings indicated the presence of a SMAD4 processed pseudogene. SMAD4 processed pseudogenes are found in 0.26% of the general population and are not considered clinically significant.2


Figure 3. SMAD4 processed pseudogene identified in SOPHiA DDM™ from A. Multiple intronic deletions called in the SNVs/INDELs tab, and B. Duplications visible in the CNV trace.
Conclusion
These three case examples demonstrate the importance of detecting CNVs in hereditary cancer samples and how the various reporting tools present in SOPHiA DDM™️ can be used in combination to successfully identify complex variants involving the PMS2 pseudogene, low-level mosaicism, and processed pseudogenes.
References
1. Vaughn CP, et al. Hum Mutat. 2010;31(5):588-93.
2. Millson A, et al. J Mol Diagn. 2015;17(5):576-82.



