Menu


Radiation pneumonitis (RP) is a significant and relatively common complication of (chemo) radiotherapy (RT) in the treatment of locally advanced non-small cell lung cancer (LA-NSCLC).
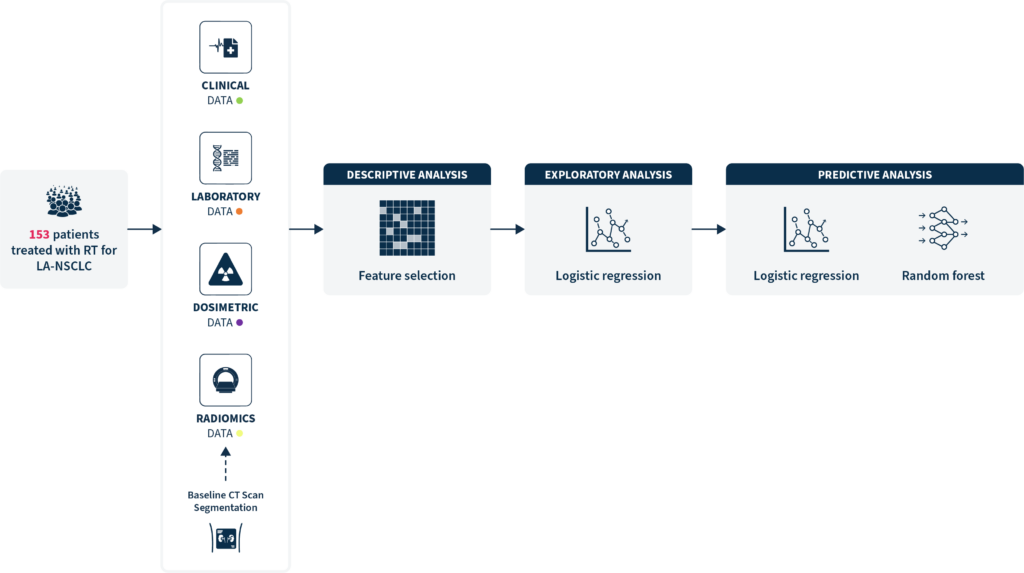
This study investigates clinical, dosimetric, and radiomic features predictive of lung toxicity, specifically grade (G)≥2 RP, in patients undergoing (chemo)RT for LA-NSCLC, with the aim to build a predictive model to estimate its occurrence.
The researchers conducted a retrospective multicenter analysis of 153 patients treated with (chemo)RT, between 2015 and 2019, to identify key risk factors. Baseline CT scans were segmented to extract radiomic features from the lungs and the tumor, and integrate them with clinical and dosimetric features.
The study employed a machine learning (ML) approach using logistic regression and random forest models to develop predictive models for RP occurrence in this patient population.
The clinical and dosimetric risk factors linked to an increased RP risk included high initial hemoglobin levels, older age, low Tiffeneau ratio (FEV1/VC), decreased initial platelet count, dosimetric factors (mean dose to lungs, lung V20Gy and V13Gy), and the use of adjuvant durvalumab.
Seven radiomic features related to intensity distribution and texture were significantly associated with RP risk.
The developed ML-based model (random forest) integrating clinical, dosimetric and radiomic data achieved the best performance with an AUC = 0.72 (95% CI [0.63-0.80]), outperforming models based on combined clinical and dosimetric data (AUC = 0.64), or on radiomic data alone (AUC=0.64).
Integrating radiomic features with clinical and dosimetric ones improves the prediction of RP, providing a more comprehensive tool for risk stratification in lung cancer patients undergoing radiotherapy.
This study showed that identifying high-risk patients for RP could allow for a more personalized treatment planning to reduce their risk, such as adjusting radiation dose constraints, introducing protective measures, or enhancing follow-up care.
Explore this infographic to learn more about this project and the predictive model developed by Evin et al’s.
Evin. C, et al. Clin Lung Cancer. 2024 Nov 20:S1525-7304(24)00248-1. doi: 10.1016/j.cllc.2024.11.003
Principal Investigator: Eleonor Rivin del Campo, MD, PhD
SOPHiA DDM™ for Radiomics and SOPHiA DDM™ for Multimodal are concepts in development. May not be available for sale.
SOPHiA GENETICS products are for Research Use Only and not for use in diagnostic procedures unless specified otherwise.
SOPHiA DDM™ Dx Hereditary Cancer Solution, SOPHiA DDM™ Dx RNAtarget Oncology Solution and SOPHiA DDM™ Dx Homologous Recombination Deficiency Solution are available as CE-IVD products for In Vitro Diagnostic Use in the European Economic Area (EEA), the United Kingdom and Switzerland. SOPHiA DDM™ Dx Myeloid Solution and SOPHiA DDM™ Dx Solid Tumor Solution are available as CE-IVD products for In Vitro Diagnostic Use in the EEA, the United Kingdom, Switzerland, and Israel. Information about products that may or may not be available in different countries and if applicable, may or may not have received approval or market clearance by a governmental regulatory body for different indications for use. Please contact us to obtain the appropriate product information for your country of residence.
All third-party trademarks listed by SOPHiA GENETICS remain the property of their respective owners. Unless specifically identified as such, SOPHiA GENETICS’ use of third-party trademarks does not indicate any relationship, sponsorship, or endorsement between SOPHiA GENETICS and the owners of these trademarks. Any references by SOPHiA GENETICS to third-party trademarks is to identify the corresponding third-party goods and/or services and shall be considered nominative fair use under the trademark law.