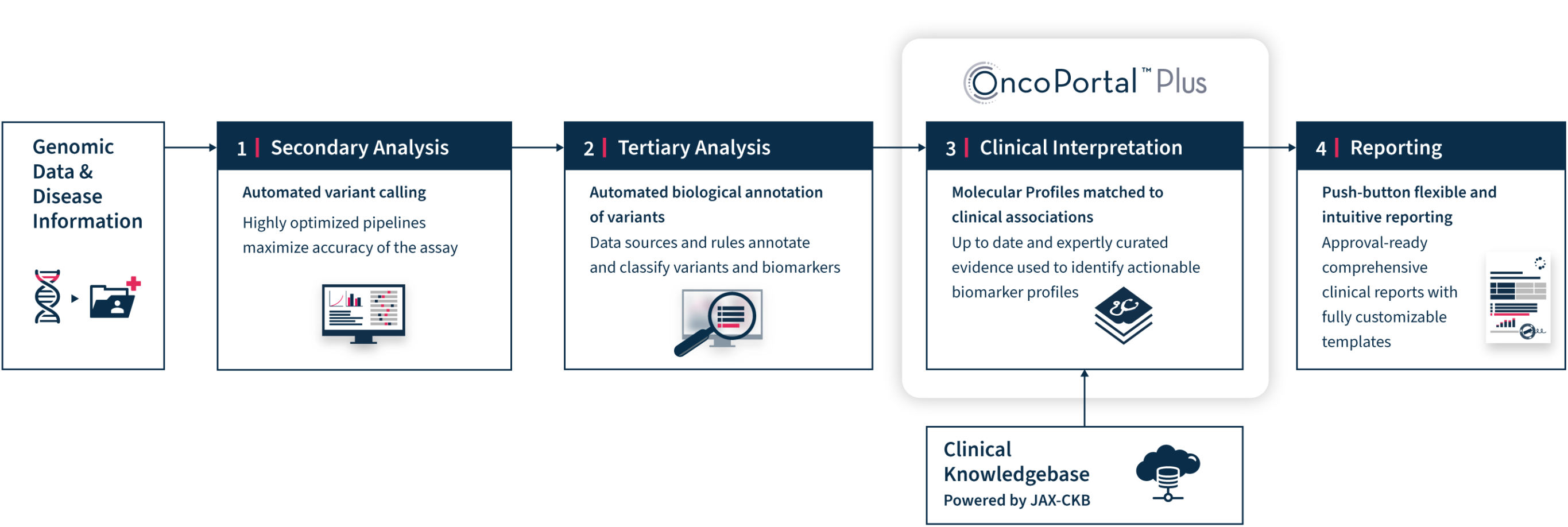
OncoPortal™ Plus
and Reporting
Confidently interpret tumor molecular profiles quickly and accurately with the latest manually-curated evidence
Manual interpretation is a thing of the past
Clinical interpretation has been one of the most complex and time-consuming aspects of transforming genomic data into meaningful results, until now.
As science evolves, more target genes are discovered, and comprehensive genomic profiling becomes standard of care, molecular laboratories must aggregate ever increasing amounts of information from multiple sources to interpret their genomic findings and prepare comprehensive reports.
OncoPortal™ Plus supports better and faster decision making with consistent and accurate interpretation of clinically significant biomarkers. OncoPortal™ Plus saves time and reduces errors that may result from manual interpretation. In tandem, our new Reporting tools enable any molecular laboratory to prepare state-of-the-art comprehensive genomic reports, while giving them the flexibility to design custom report templates to meet the unique needs of their oncologists

OncoPortal™ Plus and Reporting Workflow
Key Benefits
Accurate information
- Clinical associations, including therapeutic, diagnostic, or prognostic information support, and emerging evidence
- Transparent biomarker identification, including confidence levels
- Global clinical trial information with inclusion criteria
Consistent interpretation
- AMP/ASCO/CAP classification of biomarkers, including Tier III (VUS)
- SOPHiA GENETICS knowledge base with up-to date, expertly curated content from JAX-CKB
- Rich interpretive information with references to publications, guidelines, and regulatory approvals
Fast turnaround time
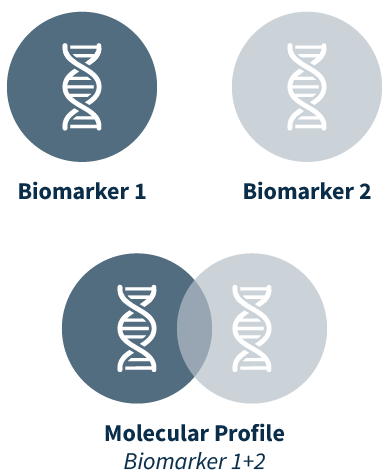
- Review co-occurring biomarkers, including wild type, in a more intuitive way by visualizing molecular profiles
- Easy to use filters to focus on the most relevant Molecular Profiles and Clinical Trials
- Free, unlimited, and customizable report templates
Product Details
A better way to interpret co-occurring biomarkers
OncoPortal™ Plus uses molecular profiles providing a better way to review and interpret biomarkers, eliminating redundant entries, and reducing the probability of missing a biologically relevant co-occurring biomarker. Rather than considering each biomarker individually, a user can quickly assess clinical associations for molecular profiles, which consider one or multiple biomarkers, including wild type, and their compound biological effect.
All biomarker types are classified according to AMP/ASCO/CAP guidelines with tiers and evidence levels. Curated interpretative text explains the gene and variant function, and clinical associations between a molecular profile and therapeutic, diagnostic, or prognostic information. Each clinical association is supported with references to original sources including literature, guidelines, and drug labels from leading global drug agencies, bringing confidence and transparency to clinical interpretation.
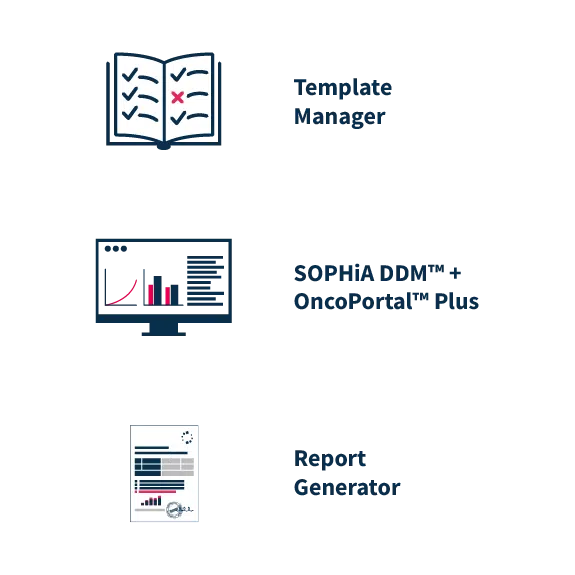
Flexibility in reporting
After clinical interpretation, the reporting tools aggregate data from OncoPortal™ Plus and SOPHiA DDM™, enabling users to prepare push-button comprehensive reports. Users can easily configure an unlimited number of custom report templates in the Template Manager to meet medical expert preferences and comply with local reporting rules and standards.
Reports are streamlined with intuitive formatting to ensure efficient communication of the findings without information overload. Case information and an overview of key insights are all found on the first page. The overview contains the actionable biomarkers, approved therapies, and a summary of other relevant biomarkers. The report is supplemented with more detailed variant information, in the form of variant cards and interpretive text. To ensure quality and consistency, all clinical associations and interpretive text is curated by a dedicated, team of experts, never crowd-sourced.
Resources
Demo Video
See OncoPortal™ Plus in action
Watch our demo video presented by Safoora Deihimi from University of Pennsylvania. See for yourself how OncoPortal™ Plus accelerates interpretation of comprehensive genomic profiles from TSO500 to learn more about OncoPortal™ Plus.
Flyer
View the flyer to see how OncoPortal™ Plus compiles all relevant clinical insights in one place to reduce time per case, increase precision, and simplify interpretation.
Sample Report
View our new intuitive formatting for yourself in our comprehensive genomic report generated with the Flexible Reporting tools in OncoPortal™ Plus.
App Note
Download our App Note. A step by step guide to variant interpretation and the features of OncoPortal™ Plus.
Want to know more?
Get in touch with us.
Disclaimer
OncoPortal™ Plus is for Research Use Only and not for use in diagnostic procedures.
OncoPortal™ Plus is an evidence-based decision support software intended as an aid in the interpretation of variants observed in genomic next-generation sequencing data. The software annotates genomic variants in the context of published biomedical literature, professional association guidelines, publicly available databases, annotations, drug labels, and clinical trials. Based on this evaluation, the software proposes a classification and bibliographic references to aid in the interpretation of observed variants. The software is NOT intended as a diagnostic tool or to be used as healthcare advice. Each laboratory is responsible for ensuring compliance with applicable international, national, and local clinical laboratory regulations and other specific accreditations requirements. OncoPortal™ Plus does not provide medical services, nor is any SOPHiA GENETICS employee engaged in the practice of medicine for or on behalf of SOPHiA GENETICS. OncoPortal™ Plus report content is for Research Use Only.
