MSK-IMPACT® powered with SOPHiA DDM™
Break the boundaries of comprehensive genomic profiling
High-performing analytics, game-changing insights
Empower your in-house solid tumor profiling research capabilities with MSK-IMPACT® powered with SOPHiA DDM™, a decentralized version of the rigorously validated comprehensive genomic profiling (CGP) test developed and used in clinical routine by Memorial Sloan Kettering Cancer Center (MSK)1.
Discover an innovative solution that combines MSK’s expertise in cancer genomics with the robust analytics of the SOPHiA DDM™ Platform for impactful solid tumor insights.
Clinical knowledge-driven design
Focus on what matters with a comprehensive 505-gene panel curated by MSK experts.
Rapid sample-to-report turnaround time
Save time with a streamlined workflow taking you from FFPE sample to report in ∼5 days.
Distinguish germline from somatic variants
Filter germline variants and reveal variants of true somatic origin with a matched tumor-normal approach.
Complex biomarker detection
Accurately detect SNVs, Indels, CNVs, DNA fusions, and more complex biomarkers including MSI and TMB.
Robust analytical performance
Trust your results with high analytical concordance to the centralized MSK-IMPACT® (e.g. 99.3% positive percent agreement for SNV/Indel calling).
Enhanced interpretation support
Leverage user-friendly interpretation features, including access to MSK’s Precision Oncology Knowledge Base, OncoKB™.
Save time with a streamlined end-to-end workflow
Equip your laboratory with a true sample-to-report workflow that combines in-house hybrid capture and cloud-based analytics, allowing you to retain control of your samples and data.

Easy library preparation and capture
505-gene panel focused on actionability, curated by MSK experts and backed by >900 publications by MSK investigators
Hybridization-based capture with tailored probes for high on-target rate and coverage uniformity
Recommended 50 ng FFPE DNA (min. 10 ng), 50 ng white blood cell gDNA
Ready-to-sequence libraries in just 1.5 days with streamlined SOPHiA DDM™ Universal Library Prep protocol
Automation options available to increase efficiency
Optimized multiplexing of paired tumor-normal samples for a cost-effective process
Compatible with NovaSeq™ 6000, NovaSeq™ X, and NextSeq® 550/1000/2000 sequencers
Advanced analysis with the SOPHiA DDM™ Platform
Algorithm-powered detection of SNVs, Indels, CNV, DNA fusions, MSI, TMB, TERT promoter, and MET exon 14 skipping
Proprietary molecular barcoding technology, CUMIN™, for sensitive variant detection down to 5% VAF
Distinguish between somatic and germline variants with user-friendly front-end features
Tertiary analysis based on the latest scientific evidence on relevant variants with OncoPortal™ Plus
Access to MSK’s Precision Oncology Knowledge Base, OncoKB™, via link-out at gene level for enhanced interpretation support
Knowledge-sharing within SOPHiA GENETICS Community, one of the largest networks of connected healthcare institutions
Complement and expand your CGP capabilities
MSK-IMPACT® powered with SOPHiA DDM™ enables comprehensive baseline characterization of tissue samples to inform precision oncology approaches. Complement your insights with:
Fusion detection from RNA
Detect partner-agnostic fusions from minimal input RNA with SOPHiA DDM™ RNAtarget Technology.
Genomic instability assessment
Identify HRD-positive samples with the universal GIInger™ algorithm for genomic instability assessment.
Liquid biopsy analysis
Facilitate rapid results and longitudinal disease monitoring with MSK-ACCESS® powered with SOPHiA DDM™.

Adapted from Mandelker D, Ceyhan-Birsoy O (2020)2.
Cut through the noise with a matched tumor-normal approach
A major limitation of tumor-only sequencing is its inability to distinguish germline versus somatic mutations2. A study showed that a tumor-only approach could not definitively identify germline changes, resulting in false positives comprising up to one third of alterations detected, including in potentially actionable genes3. Having an accurate picture of a tumor’s molecular make up is crucial for informed decision-making.
MSK-IMPACT® powered with SOPHiA DDM™ streamlines results with a matched-normal approach, allowing you to detect tumor-specific somatic variants with certainty and clearly distinguish from germline mutations associated with cancer-risk.
Trust your data
Exceptional analytical performance4
In and end-to-end comparison of 167 clinical FFPE + matched white blood cell DNA samples, MSK-IMPACT® powered with SOPHiA DDM™ achieved high analytical concordance with the centralized version used at MSK.
OPA, overall percent agreement; PPA, positive percent agreement. Concordance based on analysis between decentralized MSK-IMPACT® powered with SOPHiA DDM™ and single-site MSK-IMPACT® using 167 clinical FFPE and matched WBC gDNA samples. Limit of detection analysis based on analysis with MSK-IMPACT® powered with SOPHiA DDM™ using 216 reference samples. Analytical assessment of RUO products.

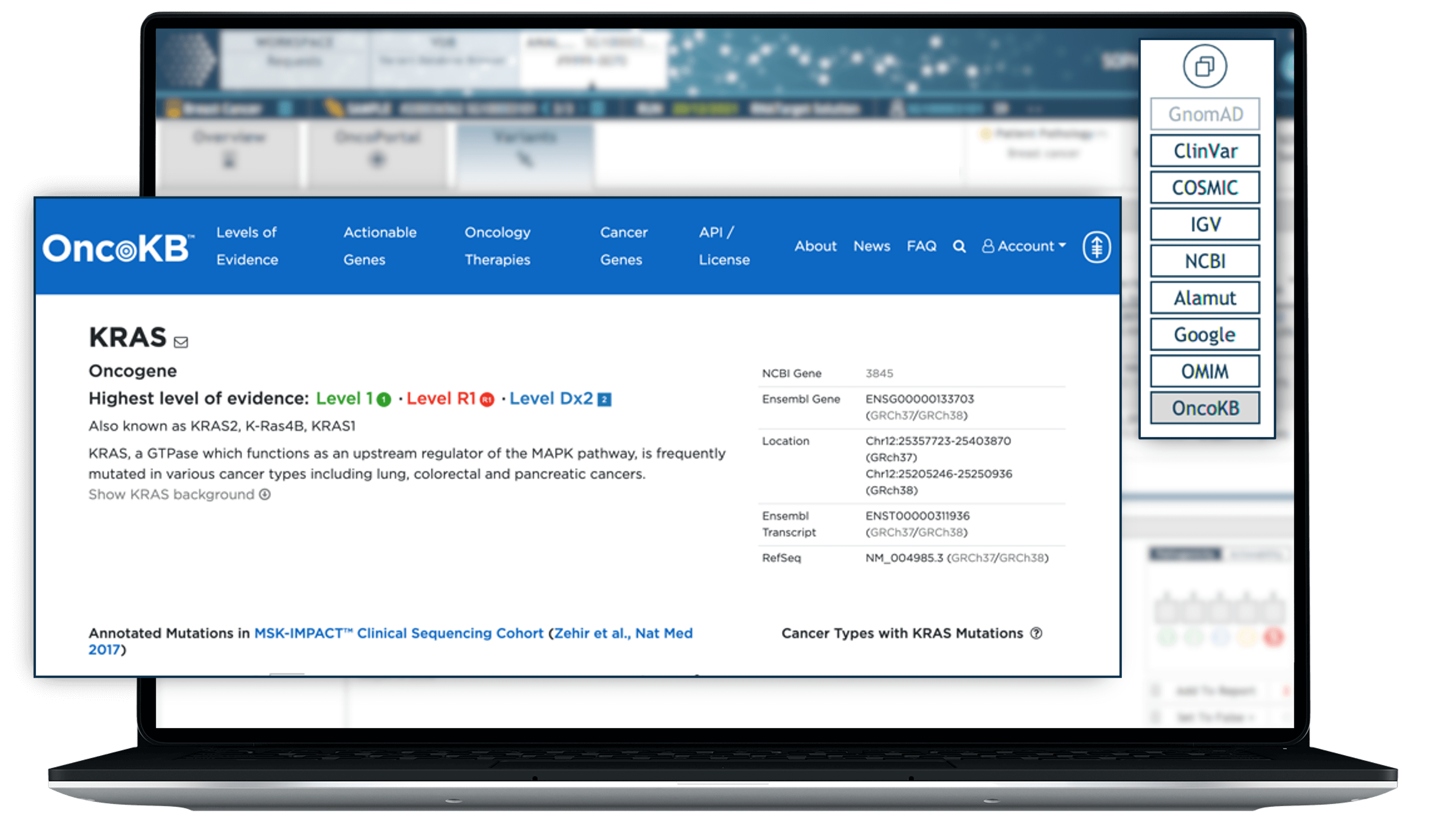
Access curated insights with OncoKB™
Enhance your somatic mutation interpretation capabilities with curated, evidence-based information offered by MSK’s Precision Oncology Knowledge Base, OncoKB™. Easily accessed via a gene-level link-out, OncoKB™ further enhances the interpretive power of SOPHiA DDM™ analysis through rich, data-driven insights.

Addressing global inequalities in comprehensive cancer care
AstraZeneca and MSK have joined forces with SOPHiA GENETICS to revolutionize cancer research and treatment. Together, we are creating a decentralized global network for precision oncology testing, including underserved regions where access remains scarce.
Generating an unparalleled and comprehensive dataset sourced from diverse populations holds the potential for invaluable insights that could shape the future of global healthcare.
Specifications
| Content | 505 genes |
|---|---|
| Diseases Covered | Multi-cancer (any solid tumor) |
| Detected Variants | • SNVs/Indels in 505 genes • CNVs (gene amplifications and deletions) in 490 genes • Intronic coverage of 23 genes for novel fusion detection • TERT promoter • MET exon 14 skipping • Tumor mutational burden (TMB) • Microsatellite instability (MSI) |
| Sample Type | Paired samples of DNA from FFPE and white blood cell (WBC) gDNA |
| Starting Material | Recommended 50 ng FFPE DNA (min. 10 ng), 50 ng white blood cell gDNA |
| Multiplexing (samples per run) | Illumina NextSeq™ 550: • High-Output: 12 (tumor + normal) pairs Illumina NextSeq® 1000/2000: • P2 flow cell: 12 (tumor + normal) pairs • P3 flow cell: 36 (tumor + normal) pairs • P4 flow cell: 48 (tumor + normal) pairs Illumina NovaSeq™ 6000: • SP: 24 (tumor + normal) pairs • S1: 48 (tumor + normal) pairs Illumina NovaSeq™ X: • 1.5B flow cell: 48 (tumor + normal) pairs |
| Library Preparation Time | 1.5 days |
Resources
Webinars
Fact sheets
Want to know more?
Get in touch with us.
References
- MSK. MSK-IMPACT®. Available at: https://www.mskcc.org/msk-impact.
- Mandelker D, Ceyhan-Birsoy O. Trends Cancer. 2020 Jan;6(1):31-39.
- Jones, S, et al. Sci Transl Med. 2015. 7(283):283ra53.
- Data on File.