Menu

We are excited to be at the 24th Annual Bio-IT World Conference & Expo. Join us at booth #724 and meet our BioPharma and Multimodal R&D experts to learn how we help BioPharma companies address challenges throughout the drug development continuum with data solutions tailored to their needs, bringing more effective drugs to the right […]
Event Type:
Event Location:
Areas of Interest:

We are thrilled to be back at 15th World Clinical Biomarkers & Companion Diagnostics Summit Europe. Join us and meet our BioPharma team to learn how we help BioPharma companies address challenges throughout the drug development continuum, enhancing efficiency and precision to accelerate the development of better and more effective precision therapeutics. If you are […]
Event Type:
Event Location:
Areas of Interest:

SOPHiA GENETICS is thrilled to be back at AACR 2025! Visit us at booth #2856 to learn how we advance data-driven medicine, with our cloud-based SOPHiA DDM™ Platform. Discover how it empowers health data interpretation with advanced analytics modules, simplified reporting, and a global community of healthcare institutions. By harnessing the power of AI and […]
Event Type:
Event Location:
Areas of Interest:

Join us at the 14th International Symposium on Minimal Residual Cancer (ISMRC) in Nice, France, where experts gather to explore the latest advancements in liquid biopsy. Discover how SOPHiA GENETICS is transforming cancer care through innovative solutions at the forefront of precision oncology. Visit our booth to learn more and connect with our team—see you […]
Event Type:
Event Location:
Areas of Interest:

SOPHiA GENETICS is thrilled Charité Mayo Conference 2025! Visit us to learn how we advance data-driven medicine, with our cloud-based SOPHiA DDM™ Platform. Discover how it empowers health data interpretation with advanced analytics modules, simplified reporting, and a global community of healthcare institutions.
Event Type:
Event Location:
Areas of Interest:

We are thrilled to announce our presence at the 3rd edition of Artificial Intelligence for Oncology. You will have the chance to meet our BioPharma and Multimodal R&D experts and learn how we help BioPharma companies address challenges throughout the drug development continuum with data solutions tailored to their needs, bringing more effective drugs to […]
Event Type:
Event Location:
Areas of Interest:

SOPHiA GENETICS is thrilled to be back at ESHG 2025! Come meet our team to learn how SOPHiA GENETICS can help turn complex generic data into life-changing Insights!
Event Type:
Event Location:
Areas of Interest:

SOPHiA GENETICS is thrilled to be back at ASCO 2025! Meet our experts at booth #32131 to hear about our biopharma solutions. Learn how we are leveraging our unique AI expertise, to provide an unparalleled offering, unlocking insights from complex data types, driving faster discovery of new stratifying biomarkers, in addition to aiding in the […]
Event Type:
Event Location:
Areas of Interest:

We are excited to be back at ECP in 2025! Join us and explore more about our recent developments in Liquid Biopsy, CGP, Solid Tumors and more!
Event Type:
Event Location:
Areas of Interest:

Join us at ESMO 2025! Find out how we advance data-driven medicine, with our cloud-based SOPHiA DDM™ Platform. Discover how it empowers health data interpretation with advanced analytics modules, simplified reporting, and a global community of healthcare institutions.
Event Type:
Event Location:
Areas of Interest:

SOPHiA GENETICS is thrilled to return for ACMG 2025! Visit us at booth #322 to learn more about our SOPHiA DDM™ and Alamut™ Visual Plus platforms for rare and inherited diseases. Exhibit TheaterFriday, March 21. 11:20 am – 11:50 am. Theatre 2 Streamlining genomic complexity with SOPHiA DDM™ and Alamut™ Visual Plus Elexandra Barboza Arguedas, […]
Event Type:
Event Location:
Areas of Interest:

As genomics research advances at an unprecedented pace, the integration of AI-powered analytics is revolutionizing germline workflows, unlocking deeper insights, enhancing data accuracy, and accelerating discoveries.
Event Type:
Event Location:
Areas of Interest:
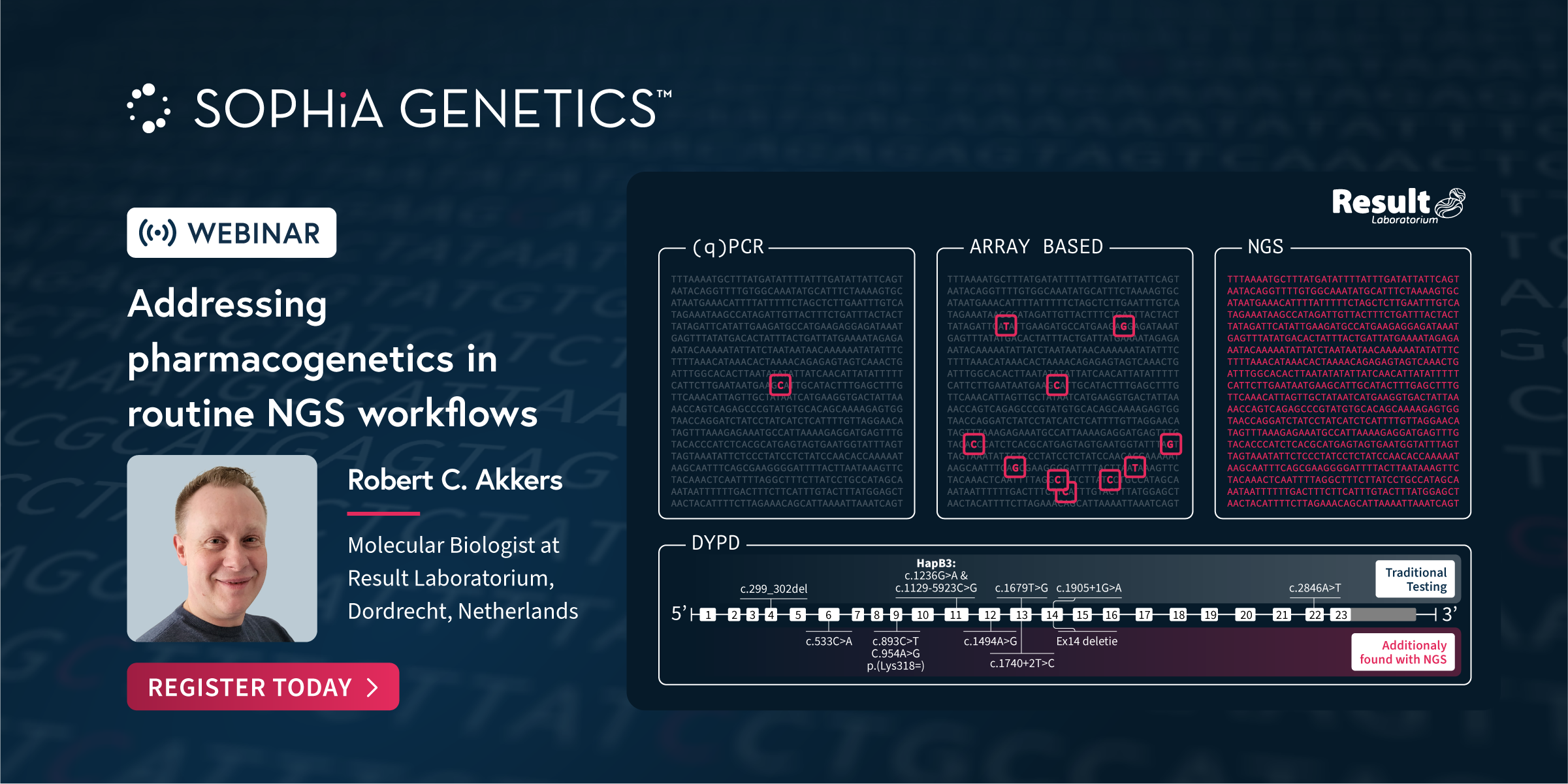
At SOPHiA GENETICS, we collaborate with genetic experts to develop specialized next-generation sequencing (NGS) applications that seamlessly integrate into any laboratory workflow. In this Webinar our partners share how the analytical technology and dedicated features in the SOPHiA DDM™️ Platform have enabled the accurate detection and streamlined assessment of variants associated with Rare Diseases and Pharmacogenomics.Discover how the […]
Event Type:
Event Location:
Areas of Interest:

SOPHiA GENETICS is thrilled to be back at this year’s Healthcare Information and Management Systems Society annual meeting. Stop by booth #3460 to meet our team and to learn how our cloud-based SOPHiA DDM™ Platform is empowering clinical researchers to break data silos and improve knowledge sharing. You can also chat with our experts and […]
Event Type:
Event Location:
Areas of Interest:

Join SOPHiA GENETICS at Pathology Update 2025 in Australia! We are thrilled to announce our participation in the Pathology Update 2025, organized by the Royal College of Pathologists of Australasia (RCPA), for the very first time! This prestigious event will take place in Australia, showcasing the theme: 'Excellence in Diagnosis.' Visit us at Booth #30 to discover how SOPHiA GENETICS […]
Event Type:
Event Location:
Areas of Interest:

Comprehensive genomic profiling (CGP) using a matched tumor-normal approach can help improve somatic detection rate and streamline interpretation.
Event Type:
Event Location:
Areas of Interest:

This webinar presents an in-depth look at how Memorial Sloan Kettering Cancer Center (MSK) is routinely using its molecular assays — MSK-IMPACT and MSK-ACCESS — together to inform precision oncology approaches.
Event Type:
Event Location:
Areas of Interest:

SOPHiA GENETICS™ is thrilled to return for Festival of Genomics! Visit us at booth #91 to learn more about the New Generation SOPHiA DDM™ Platform and Alamut™ Visual Plus.
Event Type:
Event Location:
Areas of Interest:

In this webinar, Silvia Salmoiraghi, biologist at ASST Papa Giovanni XXIII Hospital in Bergamo, Italy, discuss the performance of the SOPHiA DDM™ Residual Acute Myeloid (RAM) Solution.
Event Type:
Event Location:
Areas of Interest:

We are thrilled to announce our participation in the ESMO Asia Congress 2024! Join the SOPHiA GENETICS team at booth #E406 and explore how we are driving groundbreaking innovations in cancer research and treatment. From our collaboration with Memorial Sloan Kettering cancer Center to decentralize MSK-ACCESS and MSK-IMPACT, to our launch of SOPHiA DDM™ Residual […]
Event Type:
Event Location:
Areas of Interest:

SOPHiA GENETICS (Nasdaq: SOPH), a cloud-native healthcare technology company and a global leader in data-driven medicine, recently joined the European Liquid Biopsy Society (ELBS), a prestigious network consisting of partners from academia and industry with the common goal of making liquid biopsy tests part of the routine standard of care. SOPHiA GENETICS offers a comprehensive […]
Event Type:
Event Location:
Areas of Interest:

Pinpointing pathogenic mutations from large, complex datasets can be difficult, time-consuming, and somewhat overwhelming. So, how can you streamline your genomic analysis, to make it quicker, easier, and more efficient? In this webinar you will learn how Alamut™ Visual Plus enables clinical researchers to: ➡️ Resolve splice-site variants using splicing scores and exonic splicing enhancer […]
Event Type:
Event Location:
Areas of Interest:

Decoding Complexity – Overcoming Real-World Challenges in Variant Analysis Join us for the second episode of our webinar series, where we delve deeper into the complexities of variant analysis. Our esteemed bioinformatics experts will share practical solutions to real-world challenges in this field. Embark on a journey with us as we explore the advanced strategies […]
Event Type:
Event Location:
Areas of Interest:

Join us for an enlightening webinar on the evolution of pharmacogenetics, from its historical roots to the impact of groundbreaking innovations and the establishment of specialist foundations. We will explore the introduction of crucial guidelines and annotations that have paved the way for the development of key technologies and solutions in this field. Learn how […]
Event Type:
Event Location:
Areas of Interest:

Welcome to the inaugural episode of our new webinar series - Decoding complexity: Tackling real-world challenges in variant analysis. Prepare to embark on an enlightening journey as we tap into the wealth of knowledge possessed by our esteemed bioinformatics experts, who will be sharing practical solutions to real-world challenges in variant analysis. Each installment of […]
Event Type:
Event Location:
Areas of Interest:
Going beyond HRR mutations: A deep-learning approach on HRD detection in ovarian cancer Homologous recombination deficiency (HRD) is an important prognostic and predictive biomarker in ovarian cancer. It is assessed by combining information from homologous recombination repair (HRR) gene mutations, the “cause” of HRD, with a measure of genomic scarring, the “effect” of HRD. However, […]
Event Type:
Event Location:
Areas of Interest:

SOPHiA GENETICS is collaborating with Memorial Sloan Kettering Cancer Center (MSK) to decentralize their advanced precision oncology tools – MSK-ACCESS® for liquid biopsy and MSK-IMPACT® for comprehensive genomic profiling (CGP). By combining the clinical expertise of MSK in cancer genomics, the predictive algorithms of SOPHiA DDMTM, and the power of the global SOPHiA GENETICS network, […]
Event Type:
Event Location:
Areas of Interest:
Optimized variant prioritization for enhanced insights: SOPHiA DDM™️ and Alamut™️ Visual Plus in Action.Are you keen to improve your tertiary analysis? Discover how SOPHiA GENETICS end-to-end workflows can do just this.Our webinar covers: Presented by:
Event Type:
Event Location:
Areas of Interest:
The critical role of transcript analysis for refining the classification of variants associated with constitutional disorders. Alamut™️ Visual Plus user presentation at the ASHG 2023 Annual Meeting. Presented by: Kai Lee Yap, PhD, FACMG,Director of Molecular Diagnostics, Ann & Robert H. Lurie Children’s Hospital of Chicago, Assistant Professor of Pathology, Northwestern University Feinberg School of Medicine.
Event Type:
Event Location:
Areas of Interest:

SOPHiA GENETICS™ is excited to be a part of the American Association for Cancer Research annual meeting, held in person in Orlando, FL from April 14th-19th. You will have the chance to chat with our experts at booth #123 and learn how our BioPharma solutions may be right for you. Decentralization and Collective Intelligence: Pioneering […]
Event Type:
Event Location:
Areas of Interest:

Event Type:
Event Location:
Areas of Interest:
SOPHiA GENETICS products are for Research Use Only and not for use in diagnostic procedures unless specified otherwise.
SOPHiA DDM™ Dx Hereditary Cancer Solution, SOPHiA DDM™ Dx RNAtarget Oncology Solution and SOPHiA DDM™ Dx Homologous Recombination Deficiency Solution are available as CE-IVD products for In Vitro Diagnostic Use in the European Economic Area (EEA), the United Kingdom and Switzerland. SOPHiA DDM™ Dx Myeloid Solution and SOPHiA DDM™ Dx Solid Tumor Solution are available as CE-IVD products for In Vitro Diagnostic Use in the EEA, the United Kingdom, Switzerland, and Israel. Information about products that may or may not be available in different countries and if applicable, may or may not have received approval or market clearance by a governmental regulatory body for different indications for use. Please contact us to obtain the appropriate product information for your country of residence.
All third-party trademarks listed by SOPHiA GENETICS remain the property of their respective owners. Unless specifically identified as such, SOPHiA GENETICS’ use of third-party trademarks does not indicate any relationship, sponsorship, or endorsement between SOPHiA GENETICS and the owners of these trademarks. Any references by SOPHiA GENETICS to third-party trademarks is to identify the corresponding third-party goods and/or services and shall be considered nominative fair use under the trademark law.