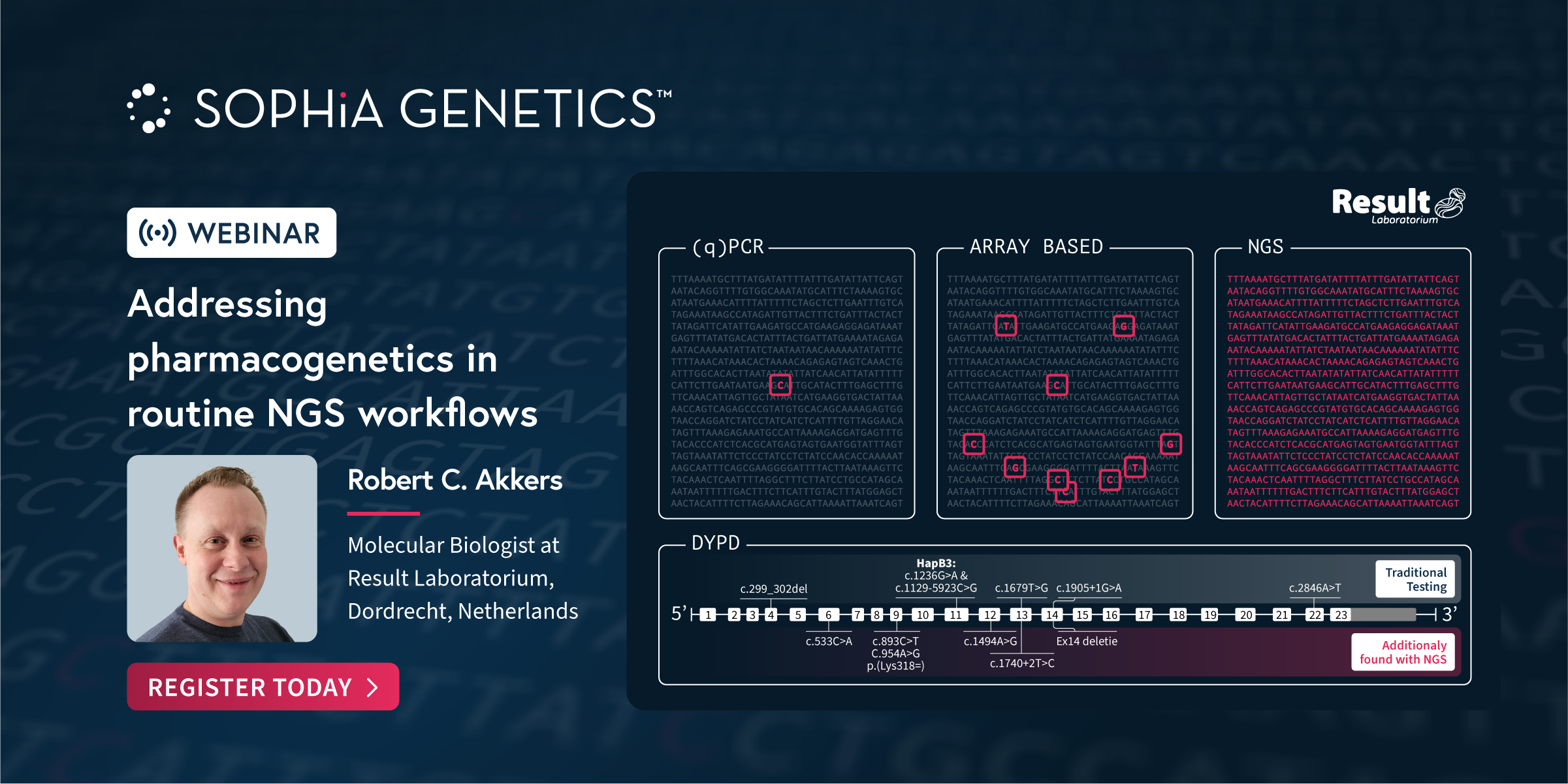
At SOPHiA GENETICS, we collaborate with genetic experts to develop specialized next-generation sequencing (NGS) applications that seamlessly integrate into any laboratory workflow. In this Webinar our partners share how the analytical technology and dedicated features in the SOPHiA DDM™️ Platform have enabled the accurate detection and streamlined assessment of variants associated with Rare Diseases and Pharmacogenomics.
Discover how the SOPHiA DDM™️ Platform seamlessly integrated into routine NGS workflows for Pharmacogenetic analysis.
This talk took place at on June 11th in Glasgow, UK at the European Society of Human Genetics (ESHG) Conference 2023.
Featured Presenter:

Robert C. Akkers
Molecular Biologist at Result Laboratorium, Dordrecht, Netherlands
After my PhD at the department for Molecular Biology in Nijmegen, I continued my career as an application specialist genomics (NGS). During this work I got really enthusiastic about the field of molecular diagnostics. After a nice postdoc position at the Department of Genetics in Wageningen I got the chance to pursue my true passion and became a molecular biologist at Result Laboratorium (Clinical Chemistry) and the Department for Pathology in Dordrecht. Besides my role in routine diagnostics I enjoy implementing new techniques and new panels for molecular diagnostics.