SOPHiA DDM™ Dx Solid Tumor Solution
High-performance profiling to optimize solid tumor management
Accurate detection and reporting of clinically relevant biomarkers are critical for better patient management.
SOPHiA DDM™ Dx Solid Tumor Solution (STS) is a CE-marked in vitro diagnostic (IVD) application based on next-generation sequencing (NGS) enabling accurate and sensitive characterization of the complex mutational landscape associated with major solid tumors. Powered by the advanced analytical capabilities of SOPHiA DDM™ Platform, this sample-to-report application helps healthcare professionals increase their efficiency and confidence of getting actionable clinical insights and making data-driven decisions that improve the quality of patient care.
Tailored analytics to accurately detect SNVs and Indels in 42 genes associated with lung, colorectal, skin and brain cancers
Reduced turnaround time with a ready-to-sequence target-enriched library in just 1.5 days
CE-IVD marked SOPHiA DDM™ Platform web for intuitive reporting with unlimited and safe data storage
Product Details
Accurately detect actionable variants
SOPHiA DDM™ Dx STS accuracy has been assessed through a multicenter performance evaluation study* showing:
- High on-target rates and coverage uniformity
- High-confidence calling of SNVs and Indels in 27 targeted genes
- Clinical-grade analytical performance that can facilitate confident decision-making.
*Performance values have been calculated based on SNVs and Indels only, in 150 samples processed in 6 clinical centers on Illumina MiSeq™.
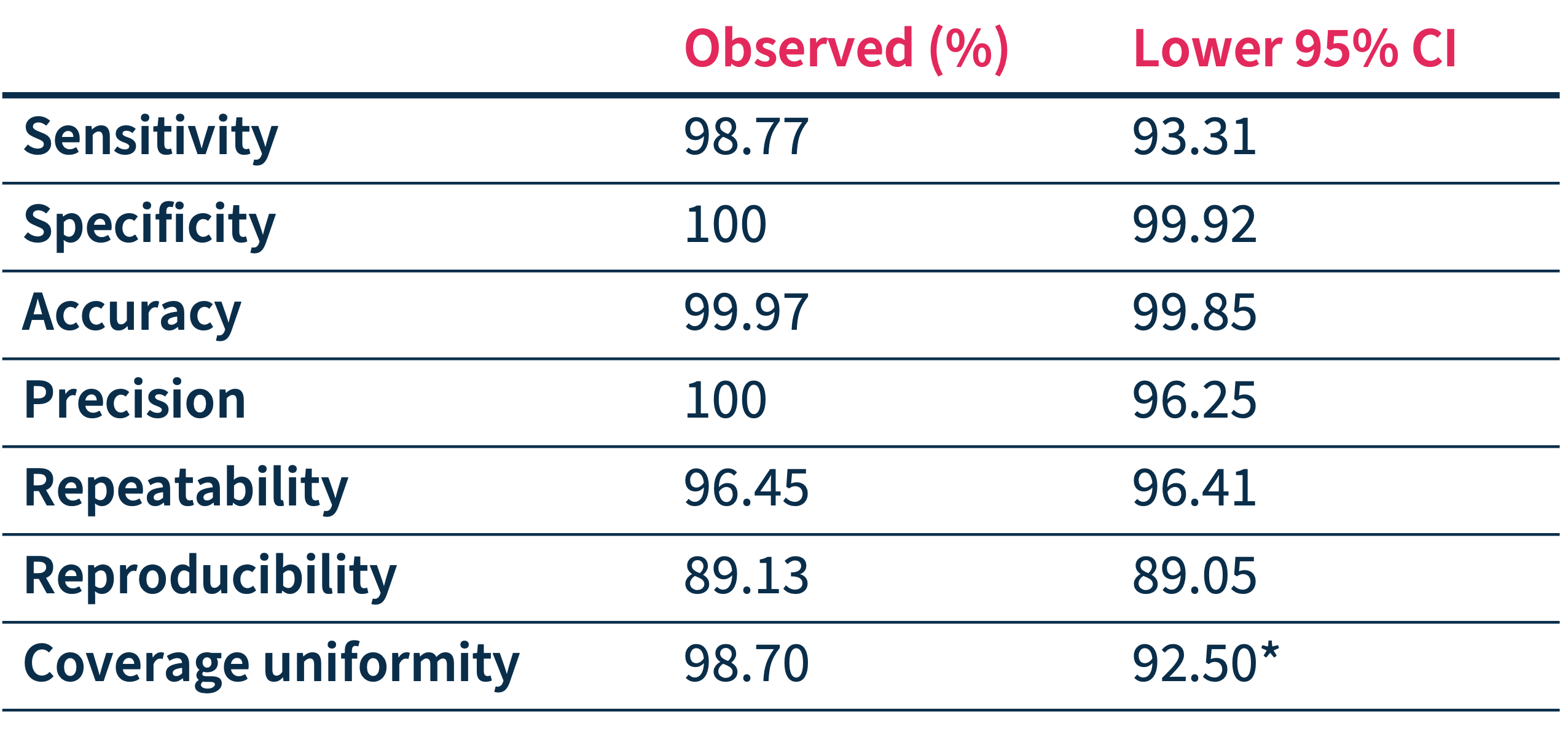
*5% quantile
Confidently generate comprehensive reports
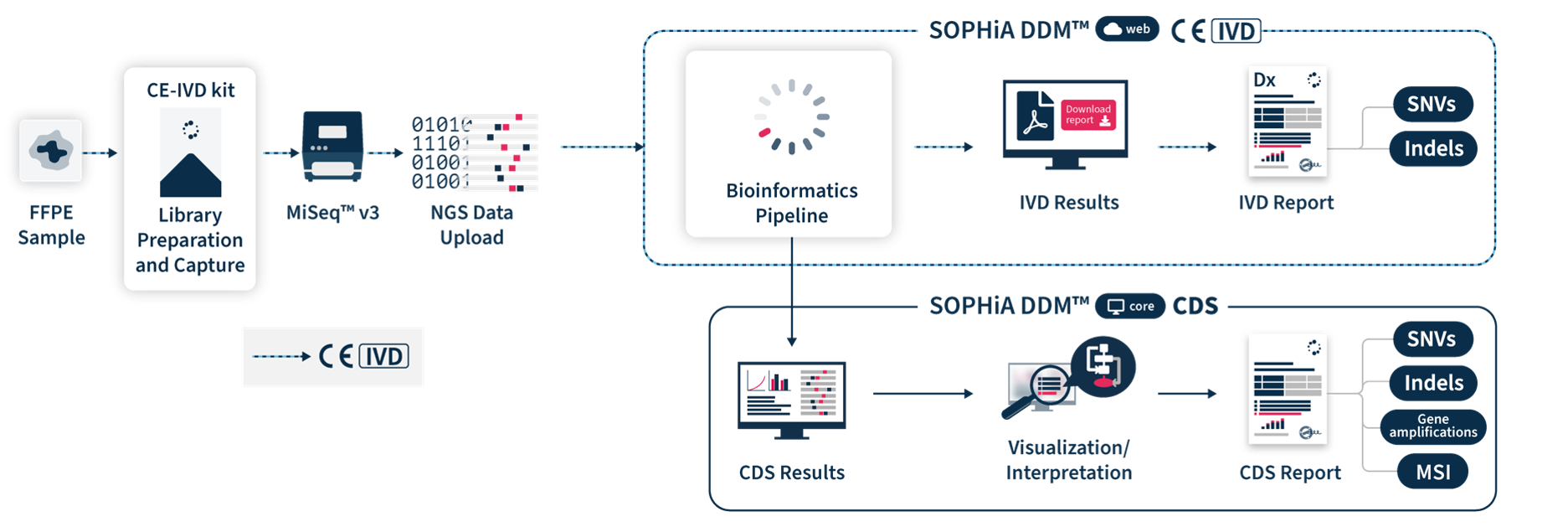
SOPHiA DDM™ Dx STS includes direct access to SOPHiA DDM™ Platform web, a front-end application to upload and analyze genomic sample data and generate downloadable CE-IVD reports. It offers unlimited and unrestricted storage, while keeping patient data safe by applying the highest industrial standards of encryption in compliance with local data security policies.
Easily interpret clinically relevant variants
SOPHiA DDM™ Dx STS offers additional insights via SOPHiA DDM™ Platform core that allows users to visualize, interpret and report key biomarkers. Clinical Decision Support (CDS*) results are computed by the bioinformatics pipeline in a single workflow. CDS* reports include SNVs, Indels and gene amplification for the 42 targeted genes and can be tuned via intuitive cascading filters and prioritization options based on databases, in-house machine learning predictions, virtual panels or custom filters. Furthermore, a Microsatellite Instability (MSI) report is provided. Users also have access to SOPHiA GENETICS™ Community to share valuable knowledge with peers.
Specifications
| Parameters | SOPHiA DDM™ Dx Solid Tumor Solution |
|---|---|
| Diseases Covered | Lung, colorectal, skin and brain cancers |
| Genes | 42 genes (AKT1, ALK, BRAF, CDK4, CDKN2A, CTNNB1, DDR2, DICER1, EGFR, ERBB2, ERBB4, FBXW7, FGFR1, FGFR2, FGFR3, FOXL2, GNA11, GNAQ, GNAS, H3F3A, H3F3B, HIST1H3B, HRAS, IDH1, IDH2, KIT, KRAS, MAP2K1, MET, MYOD1, NRAS, PDGFRA, PIK3CA, PTPN11, RAC1, RAF1, RET, ROS1, SF3B1, SMAD4, TERT, TP53) |
| Sample Type | FFPE tissue |
| DNA Input Amount | 50 ng |
| Sequencer Compatibility | Illumina MiSeq™ |
| Library Preparation Time | 1.5 days |
| Analysis Time From FASTQ | < 4 hours |
| Detected Variants |
|
| Product type | Molecular diagnostic application (kit + analytics) |
Resources
Contact us
Please fill out the form below to get in touch