SOPHiA DDM™ Dx RNAtarget Oncology Solution
Enabling novel fusion detection in small samples to improve lung cancer management
Sensitive novel fusion detection optimized for small lung cancer FFPE biopsies enhance patient care.
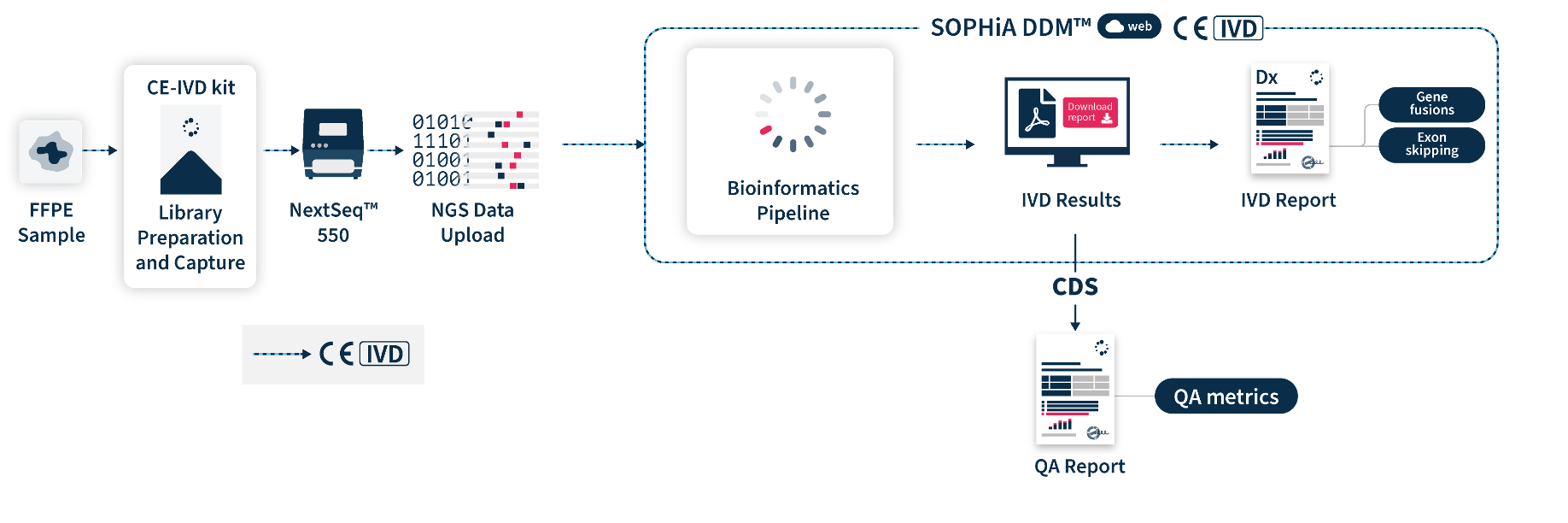
SOPHiA DDM™ Dx RNAtarget Oncology Solution (ROS) is a CE-marked in vitro diagnostic (IVD) application based on next-generation sequencing (NGS) enabling accurate and sensitive detection of novel (partner-agnostic) fusions and exon skipping events, even with minimal RNA sample input. Powered by the advanced analytical capabilities of SOPHiA DDM™ Platform, this sample-to-report application helps healthcare professionals increase their efficiency and confidence of getting actionable clinical insights and making data-driven decisions that improve the quality of patient management.
Tailored analytics to accurately detect novel fusions in 11 lung cancer-related genes as well as exon skipping in EGFR and MET
Optimized for small biopsy FFPE samples, requiring 50ng RNA input
Reduced turnaround time with a ready-to-sequence target-enriched library in just 1.5 days
CE-IVD marked SOPHiA DDM™ Platform web for intuitive reporting with unlimited and safe data storage
Product Details
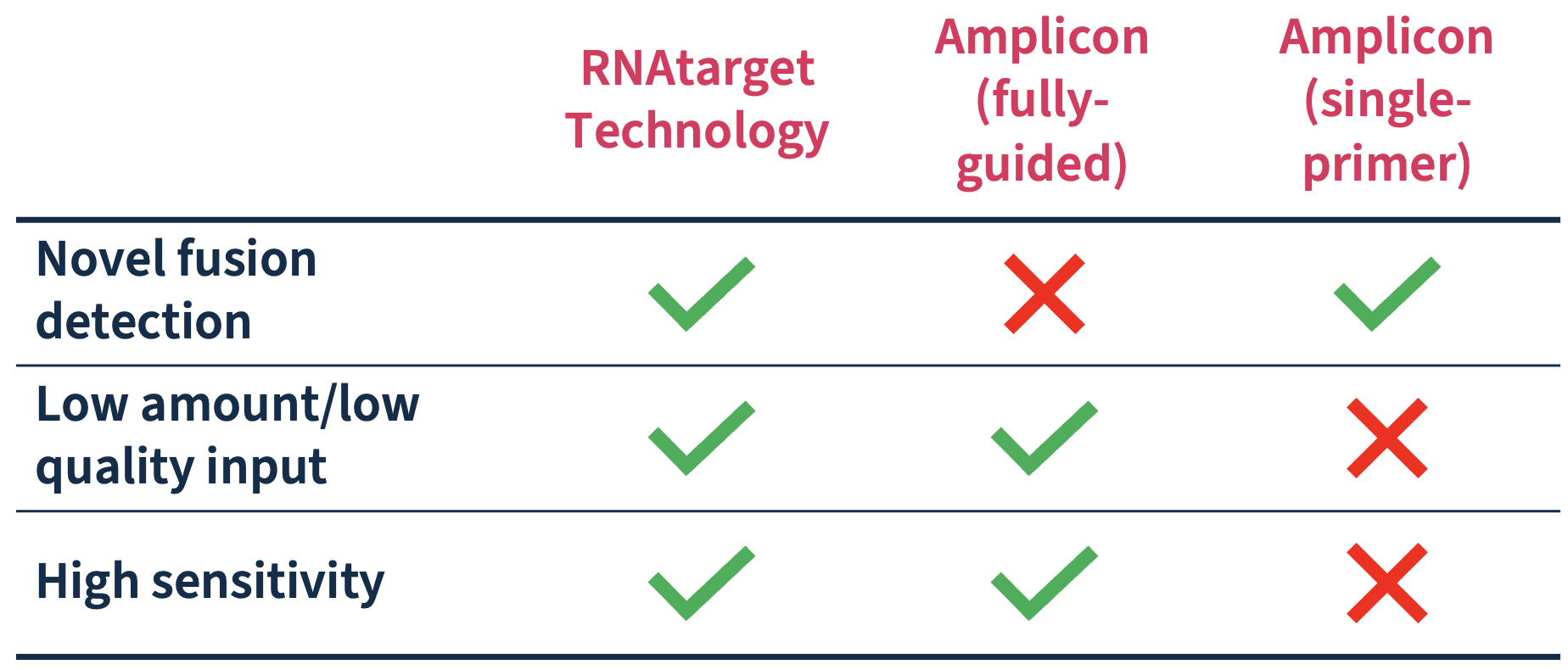
Efficiently detect novel fusions with minimal sample input
SOPHiA DDM™ Dx ROS capture-based design matches technical sensitivity of fully-guided amplicon-based solutions with only 50ng of input while being able to reduce false negatives thanks to its ability to detect fusions without prior knowledge of the partners.

**Based on the measurement of PPA/NPA in 32 replicates of a diluted reference sample, bearing 14 targeted rearrangements.
***Based on the analysis of fusion/exon skipping detection in the clinical study of 22 RNA FFPE lung tumor samples, performed by an external site.
Accurately detect fusions and exon skippings
SOPHiA DDM™ Dx ROS accuracy has been assessed through a performance evaluation study* showing:
- Reliable fusion and exon skipping detection in all targeted regions of the panel
- Clinical-grade analytical performance that can facilitate accelerated and confident decision-making.
*Status pre-determined by alternative NGS method(s) and compared with SOPHiA DDM™ Dx ROS outcome.
Confidently generate comprehensive reports
SOPHiA DDM™ Dx ROS Solution includes direct access to SOPHiA DDM™ Platform web, a front-end application to upload and analyze genomic sample data and generate downloadable CE-IVD reports. It offers unlimited and unrestricted storage, while keeping patient data safe by applying the highest industrial standards of encryption in compliance with local data security policies.
Easily interpret clinically relevant variants
SOPHiA DDM™ Dx ROS offers an additional component via SOPHiA DDM™ Platform core that allows users to obtain Clinical Decision Support (CDS*) results computed by the bioinformatics pipeline in a single workflow. CDS* feature outputs a Quality (QA) report with standard metrics computed for each sample, precious information to evaluate data quality and reinforce confidence data-driven clinical decisons. Users also have access to SOPHiA GENETICS™ Community to share valuable knowledge with peers.
Specifications
| Parameters | SOPHiA DDM™ Dx RNAtarget Oncology Solution |
|---|---|
| Diseases Covered | Lung cancer |
| Genes | Novel fusions in 11 genes (ALK, FGFR1, FGFR2, FGFR3, FGFR4, NRG1, NTRK1, NTRK2, NTRK3, RET, ROS1) and exon skipping in EGFR and MET |
| Sample Type | FFPE lung cancer tissue |
| RNA Input Amount | 50 ng |
| Sequencer Compatibility | Illumina NextSeq™ 550 |
| Library Preparation Time | 1.5 days |
| Analysis Time From FASTQ | < 8 hours / 16 sample batch |
| Detected Variants |
|
| Product type | Molecular diagnostic application (kit + analytics) |
Resources
Contact us
Please fill out the form below to get in touch