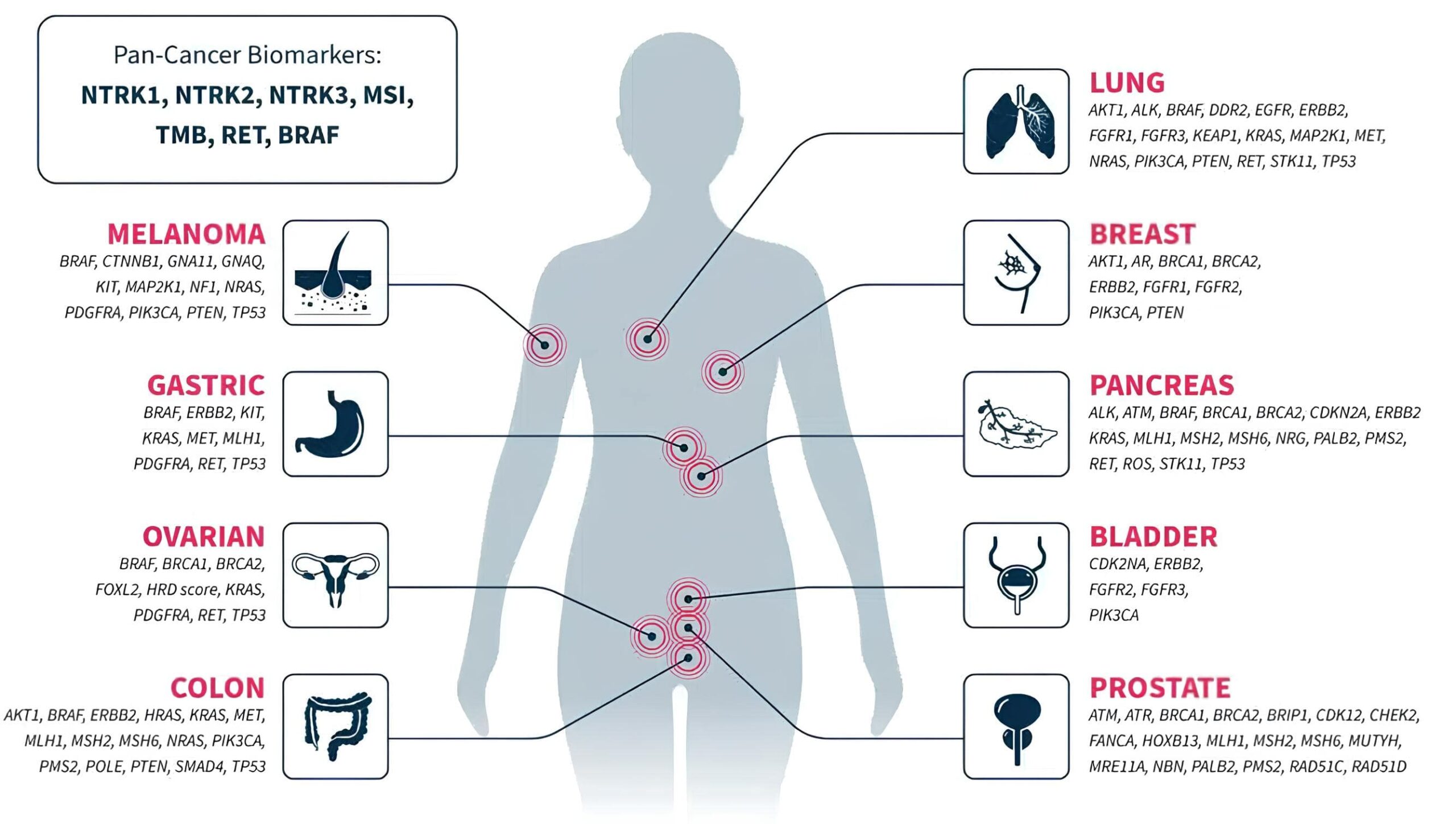
CUMIN™: Precise molecular barcoding
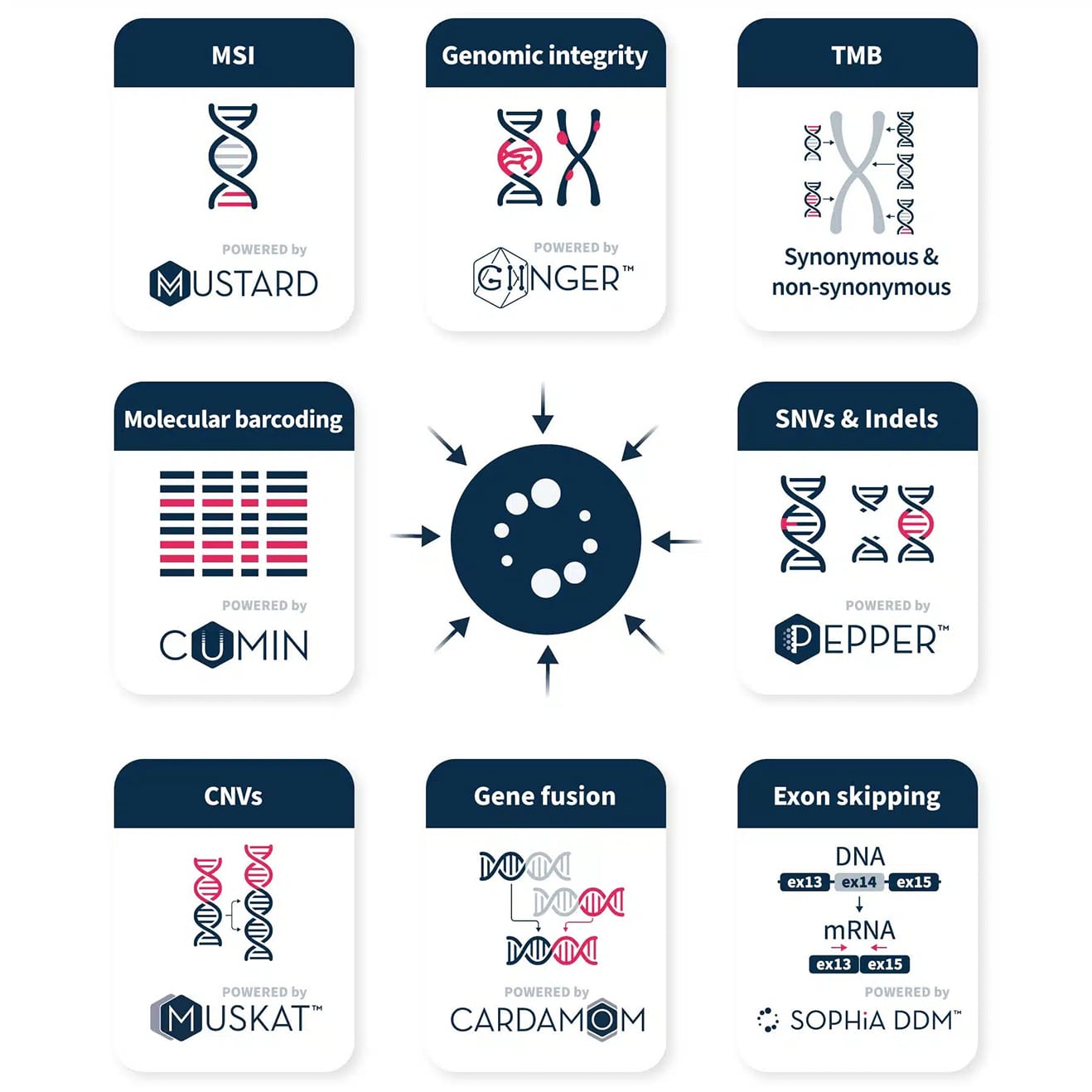
Our sophisticated SNV and Indel calling algorithm implements relevant analytical modules that are tailored to reduce noise linked to sample type, sequencer, and library preparation. The SNVs and Indels called by SOPHiA DDM™ are used to accurately estimate tumor mutational burden (TMB).
For example, our technology demonstrated high correlation for overall (R2 = 0.99) and non-synonymous (R2 = 0.99) TMB estimation when compared to a standard assay.
Technology Principles
CUMIN™: Precise molecular barcoding
Our copy number variation (CNV) calling algorithm adapts to experimental conditions and performs double normalization to call CNVs missed by other tools. MUSKAT™ accurately detects and reports whole gene amplifications and whole gene deletions.
Technology Principles
CUMIN™: Precise molecular barcoding
Our fusion calling technology accurately calls novel (partner-agnostic) fusions from DNA and RNA, utilizing a probabilistic graphical model to reduce false positives.
In analyses of RNA and tNA reference and clinical samples, CARDAMOM demonstrated 100% sensitivity (84/84 events, including 70/70 in 66 clinical samples), detecting 7 events missed by an amplicon-based approach1.

CUMIN™: Precise molecular barcoding
Apply deep learning-based analysis to low-pass WGS data (~1x coverage) in a decentralized workflow. GIInger™ is designed to complement capture-based BRCA workflows for a complete HRD assessment, with no impact on previous validation. Beyond ovarian cancer, GIInger™ adapts universally across solid tumor applications, from targeted to CGP solutions.
GIInger™ demonstrated high analytical concordance to centralized reference methods for HRD assessment in ovarian cancer (92.91% overall percent agreement)².

CUMIN™: Precise molecular barcoding
Our microsatellite instability (MSI) technology relies on a powerful curve-fitting algorithm to better identify differences in the read length distribution of MSI and normal samples.
MUSTARD™ technology demonstrated analytically accurate MSI detection in colorectal and endometrial cancer (100%), as well as in more challenging tumor types such as glioma (97.8%)³.

CUMIN™: Precise molecular barcoding
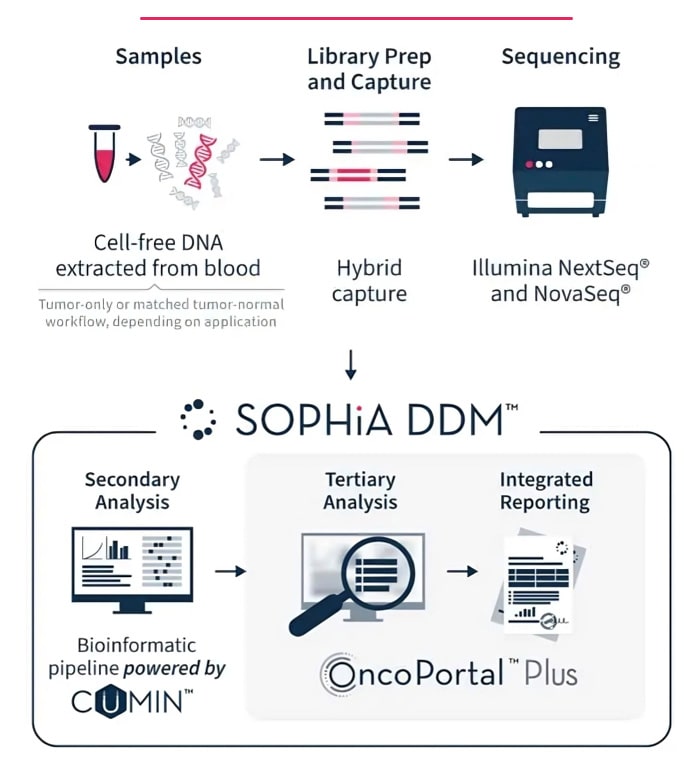
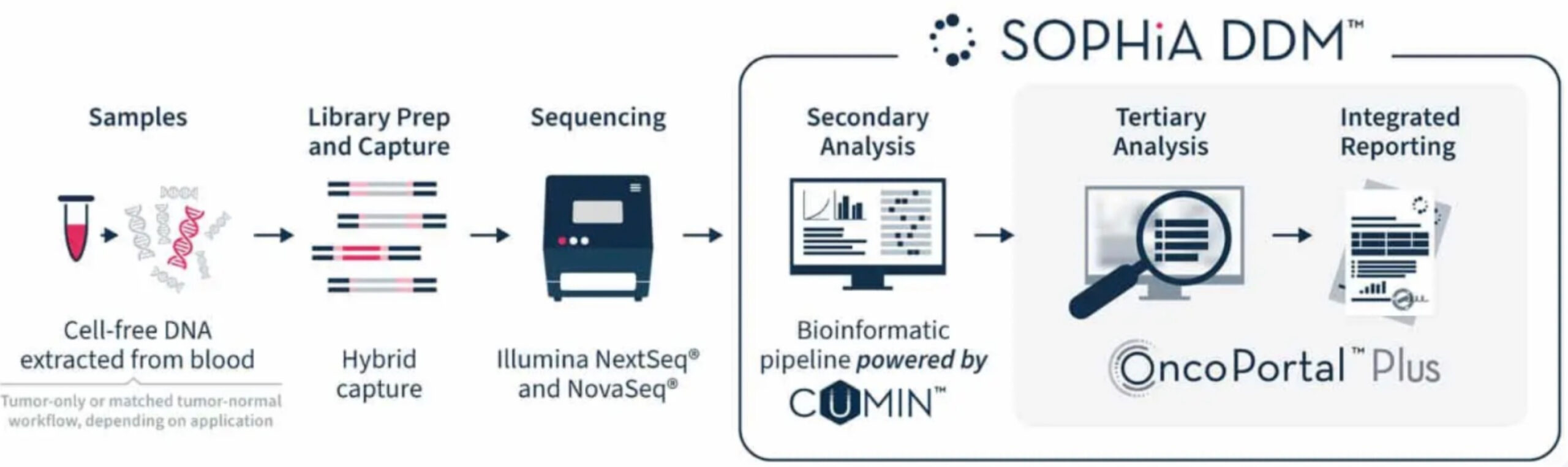
SOPHiA DDM™ enables sensitive detection of relevant variants at low allele frequencies (down to 5% VAF for FFPE anaylsis). By incorporating our proprietary CUMIN™ molecular barcoding technology into sample preparation and leveraging our bioinformatic expertise, errors introduced during library preparation, target enrichment, or sequencing can be filtered out and true variant alleles identified.
Technology Principles
CUMIN™: Precise molecular barcoding
Our annotation algorithm retrieves information from curated databases and uses de novo analytics to provide insights on the likely effects and pathogenicity of genomic variants. Coupled with SOPHiA DDM™’s filtering capabilities, our accurate variant annotation facilitates the identification of relevant variants.
Technology Principles