Case Study – Hospitale Universitario Virgen de las Nieves and Clínico San Cecilio
The Andalusian Health Service address a colorectal cancer case with the CE IVD-marked SOPHiA DDM™️ Dx Hereditary Cancer Solution.
Country
Spain
Challenge
Hereditary Cancer
Solution
SOPHiA DDM™️ Dx Hereditary Cancer Solution
The Genetics Laboratories at the Hospitale Universitario Virgen de las Nieves and Clínico San Cecilio in Granada, Spain are affiliated with the Andalusian Health Service (Servicio Andaluz de Salud) and act as reference laboratories for the Andalusian Region, primarily investigating inherited diseases and assessing hereditary cancers. The laboratories receive and analyze hereditary cancer samples from a 1.5-million-person reference population across Granada and Huelva, assessing approximately 1000 samples per year.
Research case
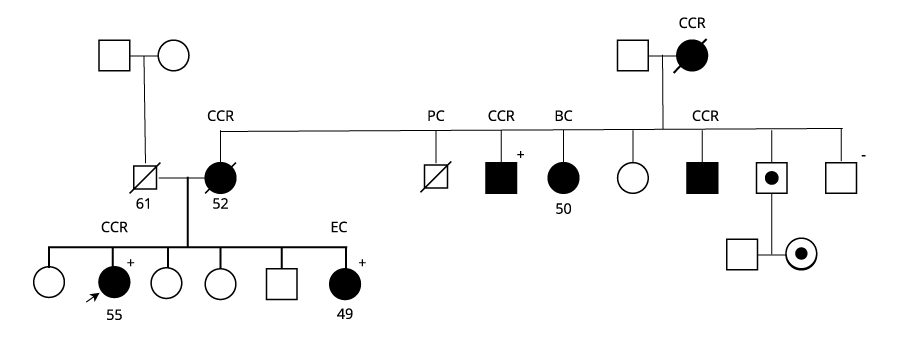
The laboratory received a sample from a man who had been diagnosed with stage II colorectal cancer when he was 55 years old. After reviewing his family history of cancer, he was later referred for genetic testing at age 60.
Pedigree. BC: breast cancer, CCR: colorectal cancer, EC: endometrial cancer, PC: prostate cancer.
Approach
The laboratory initially used immunohistochemistry to analyze the sample. Microsatellite instability was detected, along with the absence of nuclear MSH2 and MSH6, but MLH1 and PMS2 were present. The laboratory used the CE IVD-marked SOPHiA DDM™️ Dx Hereditary Cancer Solution v1.1 to further analyze the sample.
Results
The SOPHiA GENETICS™️ Application (Clinical Decision Support) enabled the discovery of a novel heterozygous duplication of exon 10 of the MSH2 gene: MSH2_ex10 2:47693693-47694052 dup. Although this duplication has not been previously reported, duplications of exons 9 and 10 have been associated with non-polyposis colorectal cancer. This information in combination with the proband’s family history led to classification of the variant as probably pathogenic. External validation by Multiplex Ligation-Dependent Probe Amplification (MLPA) was initially negative, but this proved to be due to a lack of probes in the target region. MLPA was repeated with improved probe coverage, which confirmed the mutation. These findings demonstrate the utility of the SOPHiA DDM™️ Platform’s Copy Number Variation (CNV) detection algorithm for identifying CNVs, and the need to carefully select the appropriate MLPA assay to avoid the reporting of false positives.
A segregation study was carried out among close relatives of the proband not affected by cancer, which identified 3 further carriers of the mutation in family members below age 50.
“The CNV detection algorithm in SOPHiA DDM™️ efficiently identified a complex duplication in MSH2 associated with colorectal cancer. This allowed the patient’s family members to be screened for the mutation to assess the need for further cancer risk monitoring”, says Dr. Antonio Poyatos, Hospitale Universitario Virgen de las Nieves and Clínico San Cecilio, Granada, Spain