Transform your precision medicine journey with data-driven insights
Having the right data at the right time can de-risk and accelerate your drug development journey. SOPHiA DDM™ Real-World Data (RWD) Solutions automate patient identification, streamline clinical trial site and biomarker selection, and help you visualize molecular epidemiology and genetic testing insights at a global scale. Supported by a vast clinical research network of 780+ Institutions, the SOPHiA DDM™ Platform allows you to generate data-driven evidence and leverage it to make informed decisions at every step of the process.

Discover our approach to Real-World Data exploration
The value of

Real-World Data Solutions
Leverage our global decentralized network, tailored solutions, and consultative approach to explore Real-World Data with ease.
Focus on strategic development. Leave data mining to us.
Consult with our data experts to build a solution adapted to your unique challenges and needs. Generate valuable insights in less time by identifying critical clinical data on a global scale.
Save time. Make faster, data-driven decisions.
Spend less time developing your global clinical network and instantly tap into the health data of over 750 institutions spread across 70+ countries.
Remove the guesswork. Understand your target market.
Learn about the genetic testing practices of your target market to determine the need to develop companion diagnostics, fine-tune medical education strategies, optimize your investments, and improve your go-to-market roadmaps.
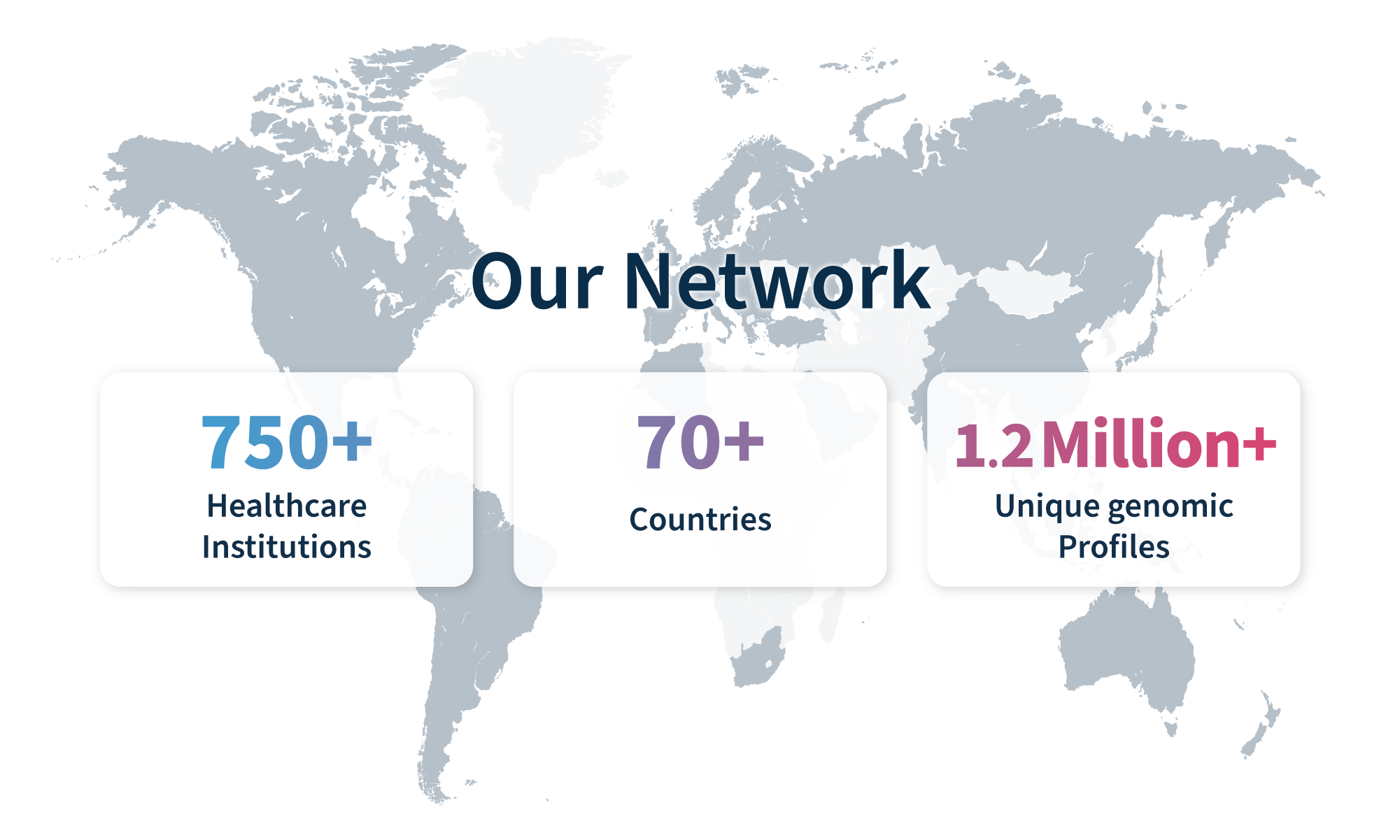
Leverage our expertise in genomics and the accuracy of the universal SOPHiA DDM™ Platform to turn complex data into valuable evidence. Drawing from 1.2 million genomic profiles across 5 continents and growing by more than 20,000 profiles every month*, our team will help accelerate your precision medicine journey and bring innovative therapies to the right markets.
*since December 31, 2021
Real-World Data Solutions that fit every step of your journey
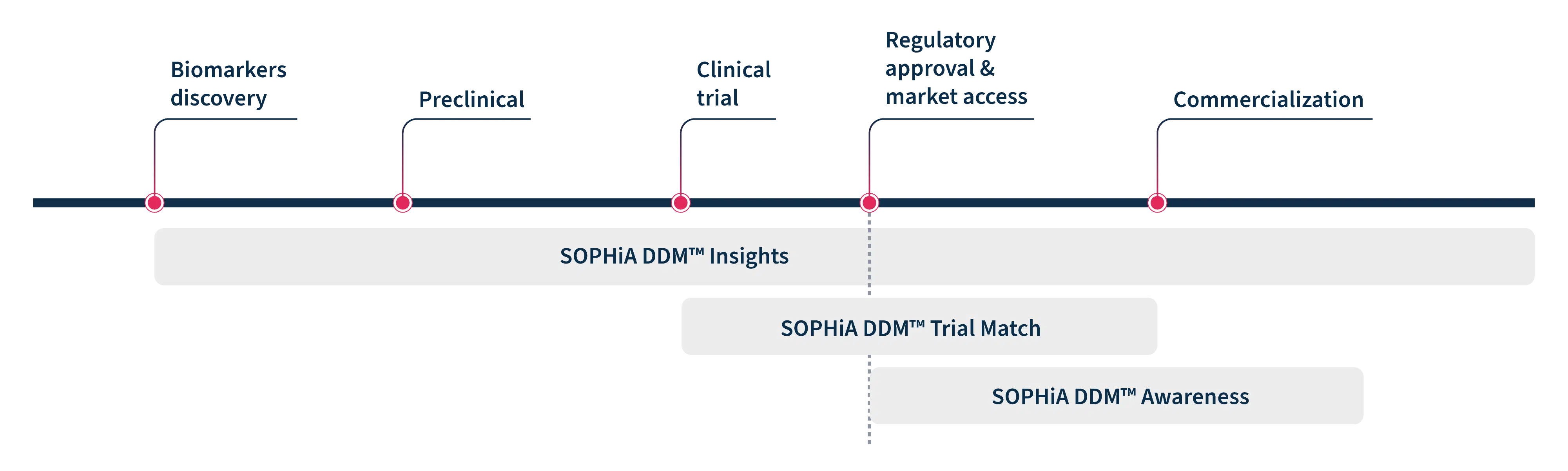
New medicines take 10+ years to be tested and approved1
From biomarker discovery to post-market surveillance, unlock valuable insights and evidence from genomic and molecular epidemiology profiles to reduce costs and accelerate your precision medicine journey.

Patient Group Identification
Understand the size and geographical distribution of your target populations and identify patient subgroups based on biomarker and molecular profiles

Biomarker Identification
Unveil co-occurring and mutually exclusive mutation patterns across diverse patient groups

Molecular Epidemiology Insights
Understand genetic alterations associated with disease at the population level
1. Marchetti S. and Schellens J. H. M. 2007. Br J Cancer. 97(5):577-581. doi: 10.1038/sj.bjc.6603925
86% of clinical trials fail to reach enrollment targets on time2
Spend less time searching for the right sites and biomarker-positive populations for your clinical trials. Shorten enrollment cycles, meet recruitment targets faster, and increase retention with on-demand access to key clinical data from across the globe.

Patient Identification
Automate real-time identification of biomarker-positive patients

Site Selection
Identify sites with large cohorts of biomarker-positive patients
Trial Rescue
Find additional sites for ongoing trials not meeting enrollment goals
2. Treweek,S., et al. 2013. BMJ Open. 3:e002360. doi: 10.1136/bmjopen-2012-002360
36% of new therapies fail to meet market expectations3
Leverage a clinical research network of over 750 institutions to generate robust and accurate commercial forecasts, optimize investments, and build sustainable, resilient, and data-centered precision medicine commercialization strategies.

Supply Chain Management
Determine the geographical distribution of biomarker-positive populations to deliver your precision therapeutics to the right target

Market Access
Determine the need to integrate companion diagnostics in your roadmap and operational plan by understanding the genetic testing landscape in your target market

Medical Education Strategy
Understand the gaps in care, knowledge, and capacity in your target market to build more engaging medical education strategies and promote precision medicine adoption
3. Ford J. et al. 2020. Key factors to improve drug launches. Deloitte Insights. Retrieved from https://www2.deloitte.com/us/en/insights/industry/life-sciences/successful-drug-launch-strategy.html